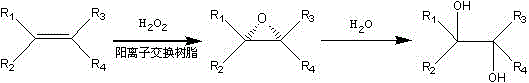
Hydrogen peroxide/cation resin system catalyzes the method of oxidizing alkenes to prepare vicinal diols
A technology of vicinal diols and resins, which is applied in the field of preparation of vicinal diols, can solve the problem of low oxidation activity, and achieve the effects of less environmental pollution, strong industrial application prospects, and a wide range of trials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 2.00g cyclohexene (0.024mol) and 2g Mn type D001 resin catalyst into a single-necked round bottom flask, keep the temperature at 70°C, slowly add 2.04g (0.012mol) 20% hydrogen peroxide dropwise, and react for 0.1- 10h, add a small amount of Na 2 S 2 o 3 Extinction reaction. The resin was removed by filtration, extracted with 10mL×4 ethyl acetate and 10mL×3 water, and the layers were separated. The water phase was crystallized at low temperature, and 2.34g of white crystals of 1,2-cyclohexanediol were obtained by suction filtration, with a yield of 84.0%.
Embodiment 2
[0021] Add 2.00g cyclohexene (0.024mol) and 10g Ag-type D001 resin catalyst into a single-necked round bottom flask, keep the temperature at 70°C, slowly add 81.62 (0.72mol) 30% hydrogen peroxide dropwise, and react for 0.1-10h , adding a small amount of Na 2 S 2 o 3 Extinction reaction. The resin was removed by filtration, extracted with 100mL×4 ethyl acetate and 100mL×3 water, and the layers were separated. The water phase was crystallized at low temperature, and 2.01g of white crystals of 1,2-cyclohexanediol were obtained by suction filtration, with a yield of 72.12%.
Embodiment 3
[0023] Add 2.00g cyclohexene (0.024mol) and 2g Mn type D001 resin catalyst into a single-necked round bottom flask, keep the temperature at 70°C, slowly add 2.72g (0.024mol) 30% hydrogen peroxide dropwise, and react for 0.1-10h , adding a small amount of Na 2 S 2 o 3 Extinction reaction. The resin was removed by filtration, extracted with 10mL×4 ethyl acetate and 10mL×3 water, and the layers were separated. The water phase was crystallized at low temperature, and 2.48g of white crystals of 1,2-cyclohexanediol were obtained by suction filtration, with a yield of 89.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com