Application of gefitinib in medicine for suppressing excessive multiplication of smooth muscle cells at injured part of blood vessel and/or promoting endothelialization of injured blood vessel
A technology of smooth muscle cells and gefitinib, which can be used in drug combinations, cardiovascular system diseases, and pharmaceutical formulations. It can solve problems such as the lack of coating drugs, so as to promote endothelialization of damaged blood vessels, inhibit excessive proliferation, and reduce thrombus. The effect formed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0124] Another aspect of the present invention also provides a method for preparing the above-mentioned gefitinib-containing drug delivery carrier. The method includes the following steps: sequentially immersing the carrier body in the drug solution, pulling it out to dry, and obtaining the gefitinib-containing drug The delivery vehicle.
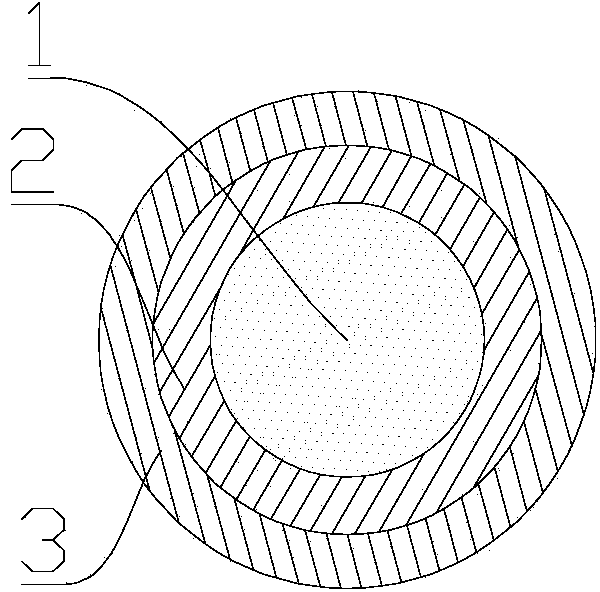
[0125] The specific steps are when the drug delivery carrier containing gefitinib is composed of a gefitinib drug layer and a polymer layer, at this time, the carrier body is first immersed in the gefitinib drug solution and left for 1-15 minutes. , Pull out the carrier, and dry the obtained carrier. Then the dried carrier is immersed in the polymer solution, soaked for 1-15 minutes, pulled out, and dried to obtain a carrier containing gefitinib. Obviously, in which order the carrier body is immersed in which solution, the drug solution of the corresponding drug layer can be selected for immersion according to the charge of the drug coating st...
Embodiment 1
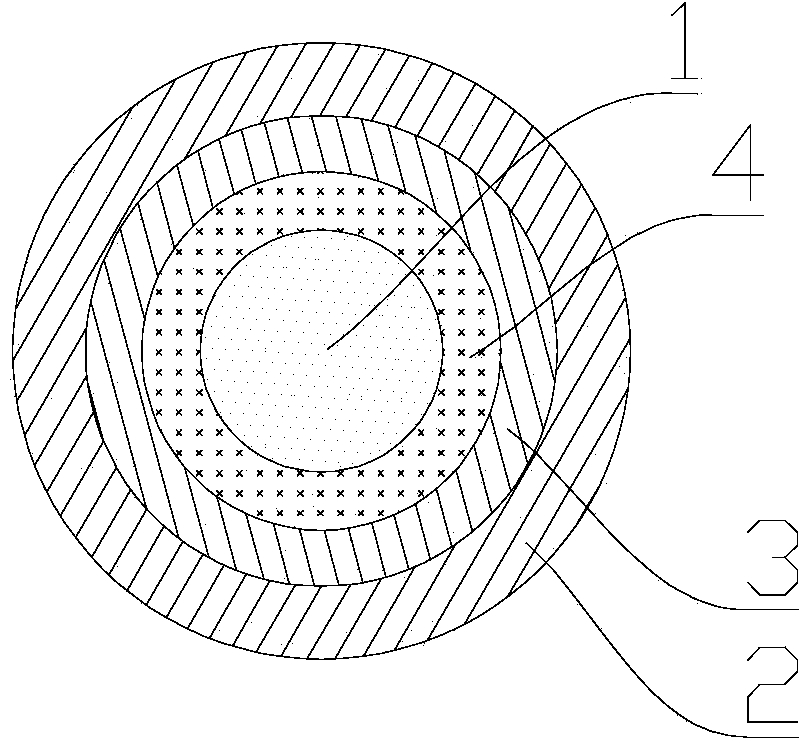
[0149] Take the nickel-titanium alloy stent and soak it in a 60℃, 20% NaOH solution for 24 hours, thoroughly clean, then ultrasonically clean in double distilled water, and dry at room temperature; dissolve PLGA to obtain a PLGA solution (the concentration of the solution is 25 / 75(mol / mol)) (molar ratio is 5%), gefitinib and PLGA mole ratio of 1:9-11 are mixed in the PLGA solution and fully stirred. The stent is immersed in the solution by dip-coating and pulling method, pulled out at a uniform speed, and then placed in a vacuum drying box to dry at room temperature until the solvent is completely volatilized. Under these conditions, a drug layer containing gefitinib GFα-SAP was prepared. Use an electrostatic spraying device to mix the solution of PLGA and heparin (using heparin sodium as heparin, the amount of heparin sodium added is 2% of the total volume of the solution; the mass of heparin on each stent is 0.35-0.50μg) (2% heparin sodium; The total amount of 0.35-0.50μg h...
Embodiment 2
[0151] Take a 3.0mm×20.0mm domestic platinum-iridium alloy stent and soak it in a 60°C, 20% NaOH solution for 24 hours, thoroughly clean it, and then ultrasonically clean it in double distilled water, and dry it at room temperature. Dissolve polylysine in PBS (phosphate buffered saline) to obtain a polycation solution with a pH of 7.2 and a mass concentration of 2.5 g / L. The above-mentioned bare alloy stent subjected to NaOH surface treatment was soaked in the polylysine solution for 30 minutes, and then taken out for later use.
[0152] Take heparin and dissolve it in double distilled water to prepare a polyanion solution with pH=4 and a mass concentration of 5g / L. Chitosan was dissolved in an acetic acid solution with a volume fraction of 1% to prepare a polycation solution with a pH of 4 and a mass concentration of 5 g / L. (The chitosan solution and heparin sodium solution used in the following examples are all prepared as described above) The gefitinib drug prepared in Exampl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com