Application of gensenoside C-K to preparing medicines for treating psoriasis
A technology of ginsenosides and C-K, which is used in drug combinations, medical preparations containing active ingredients, skin diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 : CK Antiproliferative effect on human epidermal cells in vitro
[0060] This example illustrates that ginsenosides C-K (also referred to herein as CK for short) have the effect of inhibiting the proliferation of human epidermal cells in vitro. In this example, the CK was administered to the cells by dissolving in dimethyl sulfoxide, the human epidermal cells were human immortalized epidermal cells (HaCat cells), and MTT (3-(4,5-dimethylthiazole -2)-2,5-diphenyl tetrazolium bromide salt, trade name: thiazolium blue) method to detect the survival and growth of cells.
[0061] A CK sample (commercially available, Shanghai Standard Biotech Co., Ltd. purity 92%, Lot 3690 / 20548) was dissolved in dimethyl sulfoxide to obtain a solution with a concentration of 10 mg / ml. Then use PBS for gradient dilution to obtain diluted samples with concentrations of 1000 μg / ml, 100 μg / ml, 10 μg / ml, 1 μg / ml, 0.1 μg / ml, and 0.01 μg / ml. Cisplatin for injection (freeze-dried...
Embodiment 2
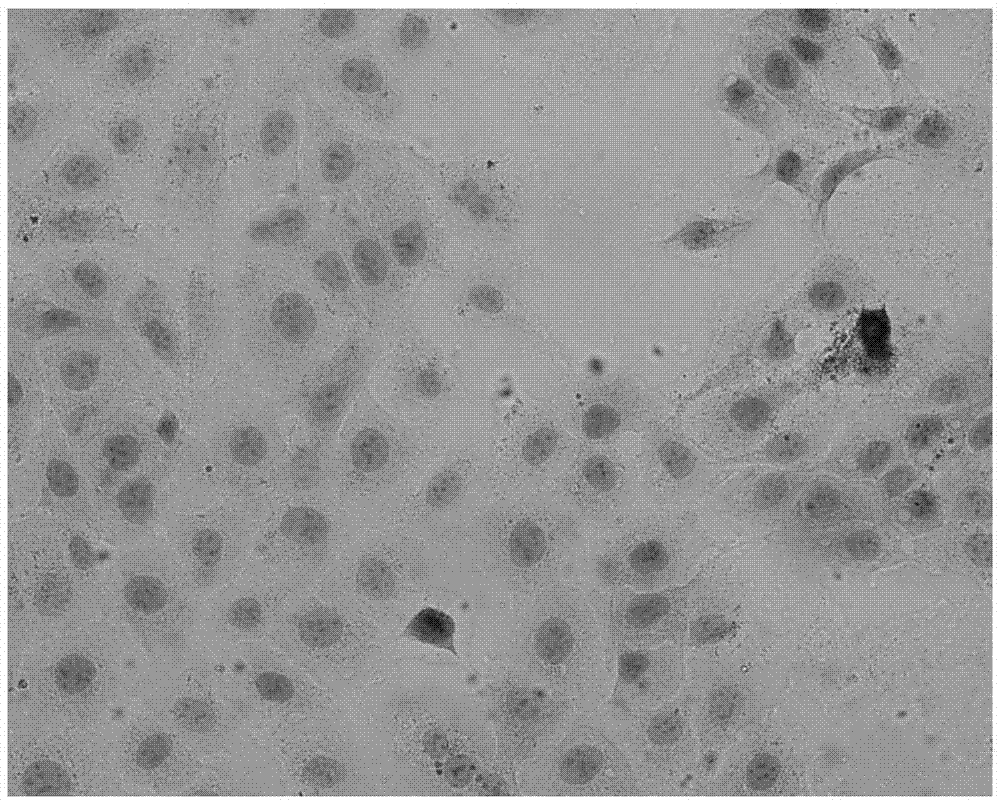
[0066] Example 2 : CK Effects on the differentiation of human epidermal cells
[0067] This example illustrates the effect of ginsenosides C-K (CK) on the differentiation of human epidermal cells. In this example, the CK was administered to the cells by dissolving in dimethyl sulfoxide, the human epidermal cells were immortalized human epidermal cells (HaCat cells), and the differentiation of the cells was detected by immunohistochemical staining.
[0068] HaCat cells were cultured in DMEM medium (containing 10% FBS), and when reaching 80% of the culture flask area, after digestion with 0.25% trypsin, the cell concentration was adjusted to 8×10 4 / ml and transferred into 6-well plates (with sterilized glass slides added to the plates), and set the concentration of ginsenoside CK to 20 μg / ml, respectively added to the corresponding wells, and set a blank control, and placed the cells at 37°C In the incubator, the anti-keratin 18 / 19 antibody (provided by the Department ...
Embodiment 3
[0070] Example 3 : CK neovascularization ( Angiogenesis of chick embryo allantoic membrane ) Has inhibitory effect
[0071] This example shows that ginsenosides C-K have inhibitory effects on chick embryo neovascularization (chick embryo allantoic membrane angiogenesis). In this embodiment, negative control substance-buffered saline (abbreviated as PBS), positive control substance-sorafenib hydrochloride (abbreviated as Sorafenib), and ginsenosides C-K were administered to chicken embryos respectively.
[0072] Sterilize the surgical instruments and filter papers used. Take fresh eggs and incubate continuously for 6 days at 37°C and 50% humidity. On the 6th day, it was identified whether the chicken embryos were viable, and the surviving eggs were taken out for window opening. First use light to locate the position of the allantoic membrane and make a mark, then make a small hole at the end of the egg air sac, place the egg with the allantoic membrane facing up...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com