Method of synthesizing saxagliptin intermediate N-t-butyloxycarboryl-3-hydroxyl-1-adamantyl-D-glycine
A technology of tert-butoxycarbonyl and adamantyl, applied in the field of organic preparation, can solve the problems of unsafe operation, environmental pollution, long process steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
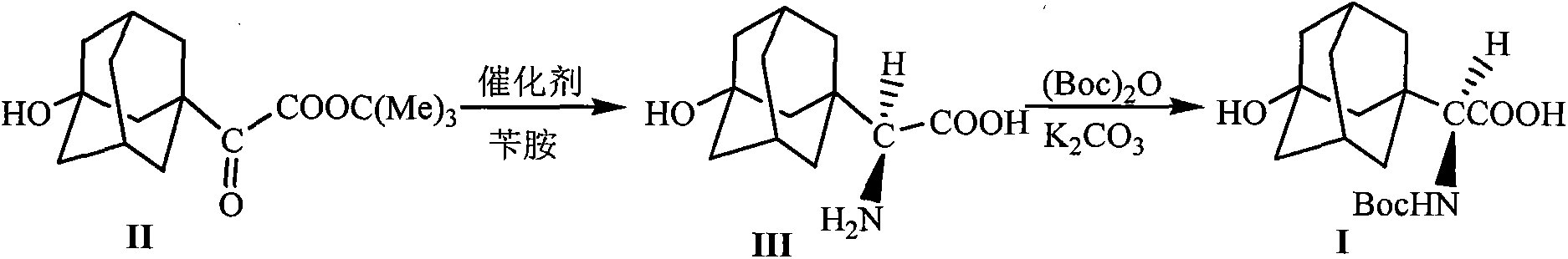
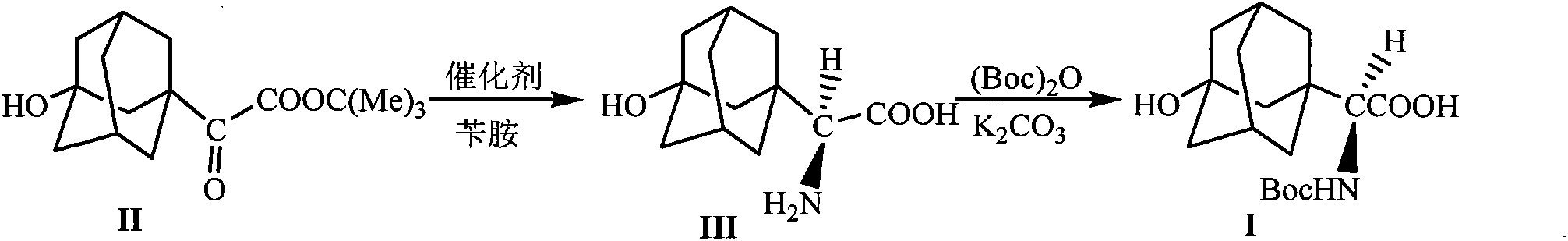
[0024] The preparation of embodiment 1 formula (III) compound 3-hydroxyl-1-adamantyl-D-glycine
[0025] 10mmol 2-(3-hydroxyl-1-adamantyl)-2-oxoacetic acid tert-butyl ester, 1.5g Molecular sieves, 10mmol o-nitroaniline and 20mL toluene were added into the reaction flask, stirred and mixed evenly. Raise the temperature of the system to 65-75°C, and stir the reaction at this temperature for 5h, stop heating, and wait until the temperature of the system drops to 40-50°C, add 0.8mmol of homemade chiral squaryl amido alcohol (R=NO 2 ) catalyst and 20mL of toluene were added, the temperature of the system was maintained at 45-55°C, and the reaction was stirred overnight at this temperature, the reaction was stopped, and filtered off after cooling Molecular sieves, add 20mL5N HCl to the filtrate at room temperature, let stand after stirring for 1h, separate the organic layer, wash with 5mL5N HCl, combine the water layer, adjust the pH value of the water layer to 8.0 with sodium car...
Embodiment 2
[0026] The preparation of embodiment 2 formula (III) compound 3-hydroxyl-1-adamantyl-D-glycine
[0027] 10mmol 2-(3-hydroxyl-1-adamantyl)-2-oxoacetic acid tert-butyl ester, 1.5g Molecular sieves, 10mmol m-chloroaniline and 20mL toluene were added into the reaction flask, stirred and mixed evenly. Raise the temperature of the system to 65-75°C, and stir the reaction at this temperature for 5h, stop heating, wait until the temperature of the system drops to 45°C, add 0.7mmol of homemade chiral squaramido alcohol (R=H) and 20mL of toluene , maintain the temperature of the system at 45-55°C, and stir the reaction overnight at this temperature, stop the reaction, filter off after cooling Molecular sieves, add 20mL5N HCl to the filtrate at room temperature, let stand after stirring for 1h, separate the organic layer, wash with 5mL5N HCl, combine the water layer, adjust the pH value of the water layer to 8.0 with sodium carbonate solid, add 90mL dichloromethane, Separate the orga...
Embodiment 3
[0028] Embodiment 3 The preparation of formula (I) compound N-tert-butoxycarbonyl-3-hydroxyl-1-adamantyl-D-glycine
[0029] Add 10mmol of 3-hydroxy-1-adamantyl-D-glycine, 0.6mmol of potassium carbonate, 12mmol of di-tert-butyl dicarbonate and 30mL of tetrahydrofuran into the reaction flask, stir and mix evenly, and react at room temperature for 12 hours. The solvent was evaporated under reduced pressure, 50 mL of petroleum ether was added to the residue, and the pH value was adjusted to 1 with 5M HCl, a white solid was precipitated, filtered by suction, and dried under reduced pressure to obtain the compound of formula (I), with a yield of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com