Synthetic method of 2, 4-dioxopiperidine
A synthesis method and a piperidinedione technology are applied in the field of synthesis of a pharmaceutical intermediate 2,4-piperidinedione, which can solve the problems of pollution, environment prone to by-products, strong corrosiveness of raw materials, etc., and achieves safe operation, The effect of few side reactions and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
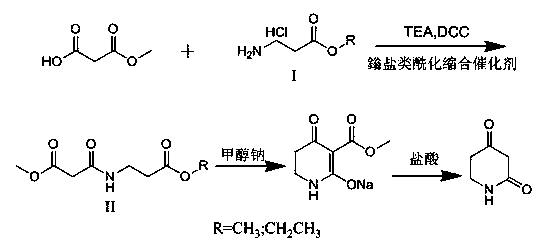
[0025] Embodiment 1: Compound I is 3-alanine methyl ester hydrochloride,
[0026] (1) Acylation: Add 150g of dichloromethane to the reaction bottle, add 20g of compound I under stirring, then lower the temperature in the reaction bottle to 10°C, add 35g of triethylamine, after the addition, control the temperature to 10 °C, 17.6g of raw material 1, 25g of DCC, and 1.5g of HATU were sequentially added, and the mixture was kept at 10°C and stirred for 20 hours, and the reaction was completed.
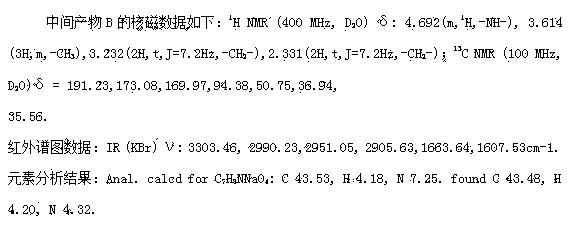
[0027] Post-treatment: first filter out the solid DCC in the reaction solution, wash the filtrate with dilute hydrochloric acid with a pH of 5, dry with anhydrous sodium sulfate, and evaporate the solvent to obtain the compound II product as a light yellow liquid.
[0028] (2) Condensation: Dissolve the compound II prepared above in 60 g of toluene solution, add 6.8 g of sodium methoxide solid to the reaction liquid at a time at room temperature, heat the mixture under reflux for 1 hour, ...
Embodiment 2
[0030] Embodiment 2: Catalyst adopts HBTU, and compound I is 3-alanine methyl ester hydrochloride,
[0031] (1) Acylation: Add 120g of dichloromethane to the reaction bottle, add 25g of compound I under stirring, then lower the temperature in the reaction bottle to 8°C, add 30g of triethylamine, after the addition, control the temperature to 8 ℃, add 16g raw material 1, 20gDCC, 1.4gHBTU successively, keep the mixture at 8℃ and stir for 18h, and the reaction ends.
[0032] Post-processing: first filter out the solid DCC in the reaction solution, wash the filtrate with dilute hydrochloric acid with a pH of 5, dry with anhydrous sodium sulfate, and evaporate the solvent to obtain 30.6 g of compound II, which is a light yellow liquid.
[0033] (2) Condensation: Dissolve the compound II prepared above in 62.5 g of toluene solution, add 6.0 g of sodium methoxide solid to the reaction liquid at a time at room temperature, and heat the mixture under reflux for 1.5 h until the reaction...
Embodiment 3
[0035] Embodiment 3 catalyst adopts pyBOP, and compound I is 3-alanine methyl ester hydrochloride,
[0036] (1) Acylation: Add 100g of dichloromethane to the reaction bottle, add 20g of compound I under stirring, then lower the temperature in the reaction bottle to 5°C, add 37.5g of triethylamine, after the addition, control the temperature to At 5°C, 20g of raw material 1, 16g of DCC, and 1.25g of pyBOP were sequentially added, and the mixture was kept at 5°C and stirred for 18 hours, and the reaction was completed.
[0037] Post-treatment: first filter out the solid DCC in the reaction solution, wash the filtrate with dilute hydrochloric acid with a pH of 5, dry with anhydrous sodium sulfate, and evaporate the solvent to obtain 32.1 g of compound II, which is a light yellow liquid.
[0038] (2) Condensation: Dissolve the compound II prepared above in 75g of toluene solution, add 7.5g of sodium methoxide solid to the reaction solution at a time at room temperature, and heat t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com