Utilizing a multiphase reactor for the conversion of biomass to produce substituted furans
A multiphase reactor and biomass technology, applied in organic chemistry, fermentation, etc., can solve the problems of slow kinetics, reaction conditions and biofuel preparation methods, and achieve cost-effective results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
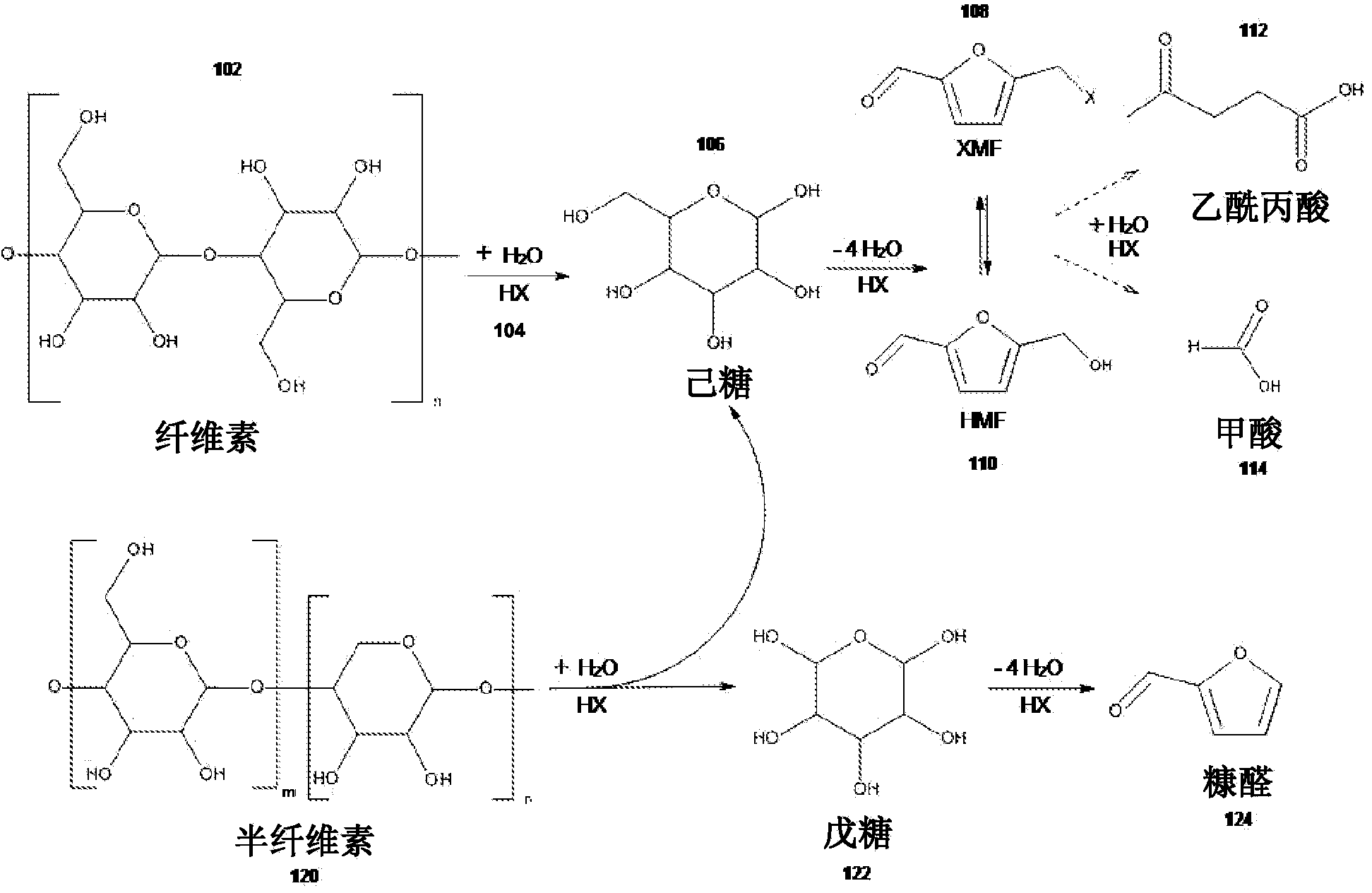
[0112] Example 1: Preparation of 5-(chloromethyl)furfural and 5-(hydroxymethyl)furfural from lignocellulosic biomass and furfural
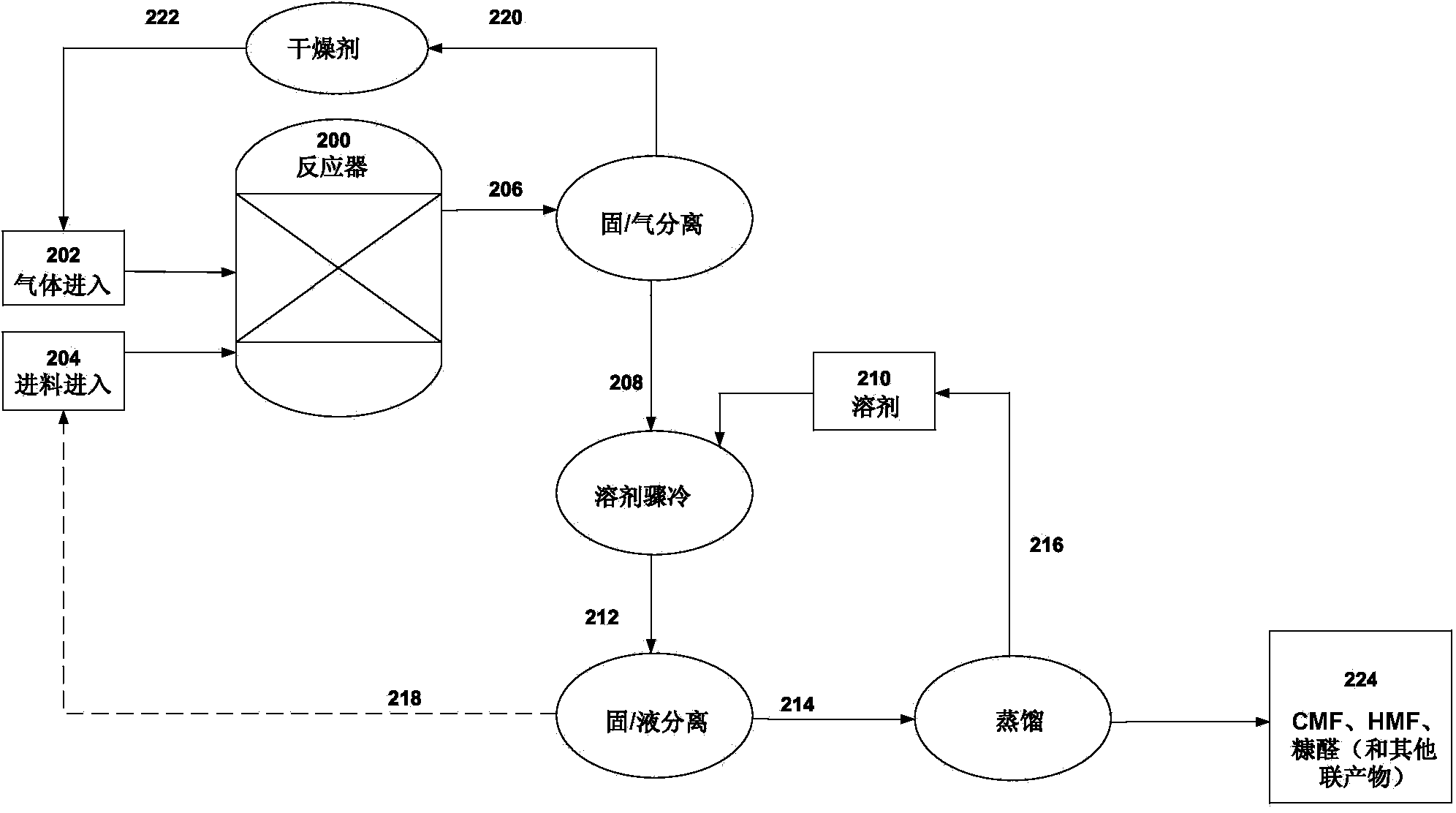
[0113] Lignocellulosic biomass was obtained from municipal wastewater in Davis (CA) and fed into a 2000-L fluidized bed reactor via side feed at a rate of 50 kg / min. Gaseous hydrochloric acid was fed into the reactor at 4,000 L / min via the other side input. The temperature inside the reactor was about 220°C, and the pressure inside the reactor was about 15 atm.
[0114] Fluidization occurs inside the reactor so that the sludge and gaseous acid can be thoroughly and uniformly mixed. After a residence time of two minutes, the crude reaction mixture of gas and solids reaches the top of the reactor and exits the reactor due to the pressure difference inside (about 15 atm) and outside (about 4 atm) of the reactor. As the gas and solids leave the reactor, the crude reaction mixture is separated into gaseous hydrochloric acid and a solid / liquid mix...
Embodiment 2
[0118] Example 2: Preparation of 5-(chloromethyl)furfural (CMF) from fructose
[0119] Into a clean and dry 350 ml pressure-sealed round bottom flask equipped with a magnetic stir bar was added granulated fructose (0.75 g, 4.16 mmol). After that, the flask was placed under an inert argon atmosphere, and dry calcium chloride (5.6 g, 50.4 mmol) and aluminum trichloride (0.119 g, 0.832 mmol) were added, and the solid was suspended in 1,2-dichloroethane (75ml). The reaction mixture was removed from the inert atmosphere and gaseous hydrochloric acid (0 wt% in water) was bubbled into the solution until gas started to come out of the flask. The flask was then sealed and placed in a preheated oil bath at 85°C and allowed to stir for 1 hour. Then, the reaction mixture was cooled to room temperature. The solid was filtered through filter paper and diluted into 100ml of solvent. Volumetric analysis using a CMF flame ionization detector (FID) - standard curve showed a concentration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com