Method for separating and recovering iron from red mud
A technology of separation and recovery and red mud, applied in the field of comprehensive utilization of waste red mud, can solve the problems of slow decomposition rate of ferric oxalate solution in sunlight, large consumption of reagents, pollution, etc., to achieve resource utilization, simple and thorough separation , the effect of reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
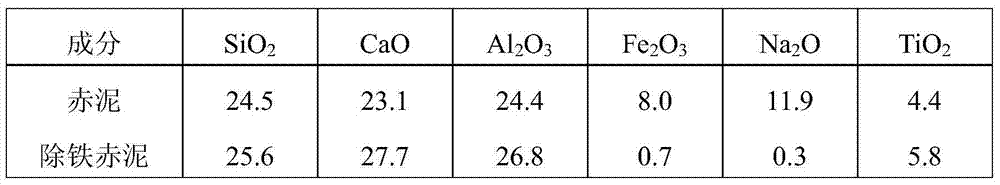
[0037] Take 100g of red mud, add water to slurry according to the solid-to-liquid ratio of 1:4g / mL, and add sulfuric acid to adjust the pH value of the solution to 6.5, stir and wash at 50°C for 0.5h, filter, and add 2000ml of oxalic acid with a concentration of 0.5mol / L to the filter cake In the solution, stirring and leaching at room temperature for 4 hours, filtering to obtain iron-removing red mud and leachate containing iron oxalate. Sampling and testing after drying the iron-removing red mud, adding CaO / CaCO to the leaching solution containing iron oxalate 3 A mixture with a molar ratio of 1:6, adjust the pH of the solution to 7.5, stir at 40°C for 1 hour, and filter to obtain a mixed filter residue and filtrate of calcium oxalate and ferric hydroxide. The filtrate is returned to prepare the red mud leachate, and the filter residue is first added with saturated CaCl with a HCl concentration of 2mol / L at a solid-to-liquid ratio of 1:1g / ml 2 solution, after slurrying for ...
Embodiment 2
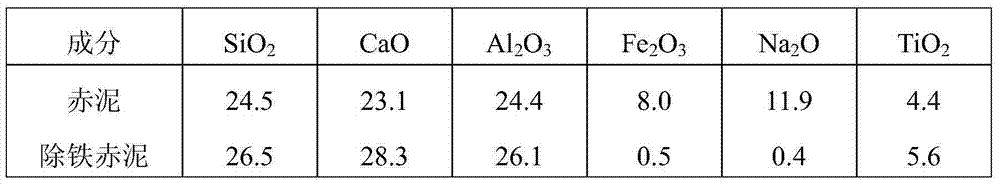
[0040] Take 100g of red mud, first add 100ml of sulfuric acid solution with a concentration of 1mol / L, then add 1000ml of oxalic acid solution with a concentration of 1.5mol / L, stir and leach at 50°C for 2.5h, and filter to obtain iron-removing red mud and leachate containing iron oxalate. Sampling and testing after iron removal red mud was dried, leachate containing iron oxalate was used as catholyte, sulfuric acid-sodium sulfate saturated solution was used as anolyte, control cell voltage was 3.7V, current density was 0.8A / cm 2 , membrane electrolysis, ferrous oxalate is precipitated in the catholyte. The ferrous oxalate obtained by filtration is mixed with 6mol / L hydrochloric acid solution at a solid-to-liquid ratio of 1:3g / ml, stirred at 65°C for 0.5h, cooled to 5°C, stirred for 5h to crystallize oxalic acid, filtered to obtain oxalic acid crystals and solution of ferrous chloride. The obtained oxalic acid is used to prepare red mud leachate. Add ammonia water to the sol...
Embodiment 3
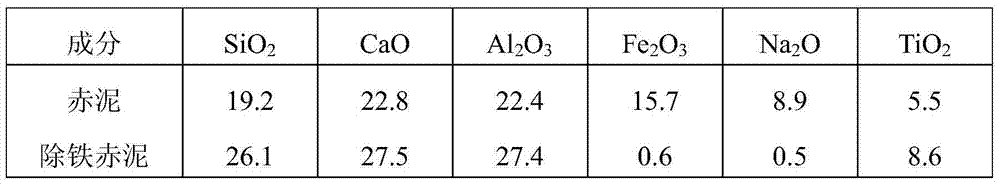
[0043] Take 100g of red mud and add 1500ml of oxalic acid solution with a concentration of 1mol / L, stir and leach at 80°C for 1h, filter to obtain iron-removing red mud and leachate containing iron oxalate. After drying the red mud for iron removal, take a sample for analysis, add iron powder to the leaching solution containing iron oxalate according to 1.1 times the stoichiometric number of Fe(III) reduced to Fe(II), stir at room temperature for 3 hours, precipitate ferrous oxalate, filter, The ferrous oxalate filter residue and the solution after reducing iron precipitation are obtained. The resulting reduced iron-precipitated solution is returned to prepare red mud leaching solution, and the obtained ferrous oxalate filter residue is added to 6mol / L sulfuric acid solution at a solid-to-liquid ratio of 1:4g / ml, stirred at 95°C for 0.5h, and filtered while hot to obtain sulfuric acid Ferrous filter residue and mixed solution containing sulfuric acid and oxalic acid. After th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com