4,2',4'-triethoxy-5' substituted chalcone derivatives and preparation method and application thereof
A technology of trimethoxy and chalcone is applied in the field of new compound synthesis and pharmaceutical application, and achieves the effects of high yield, mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
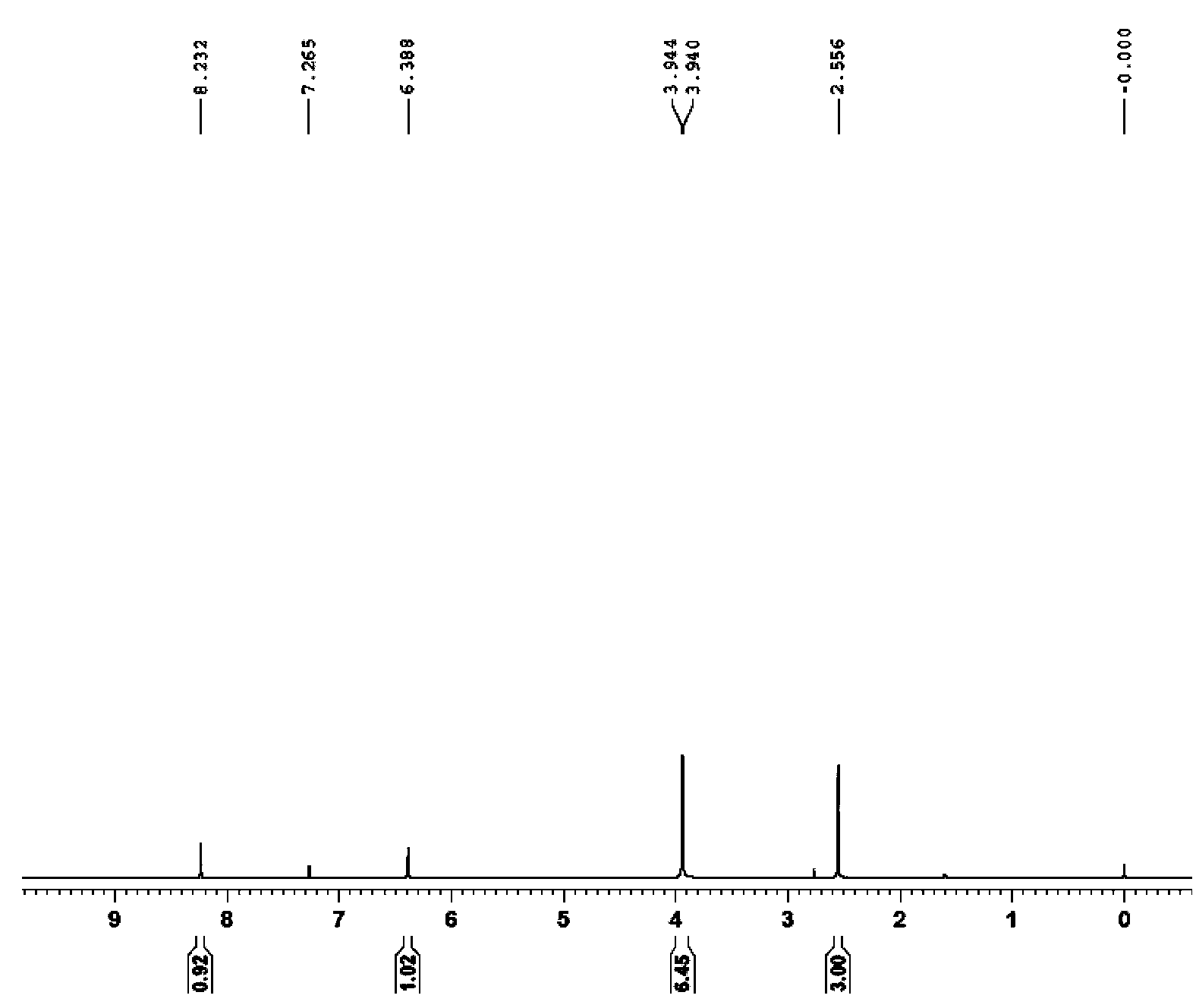
[0036] R is p-methoxyphenyl, that is, 4,2',4'-trimethoxy-5'(p-methoxyphenyl)chalcone. The preparation steps are as follows
[0037] (1) Synthesis of intermediate 2,4-dimethoxyacetophenone
[0038] AlCl 3 (25g, 159.23mmol) was dissolved in 150mL of anhydrous dichloromethane, the system was protected with argon, and acetyl chloride (15g, 188.18mmol) was slowly added dropwise under ice bath conditions with constant pressure drops, until all the acetyl chloride was added dropwise After completion, m-xylylene dimethyl ether (20 g, 144.8 mmol,) was dissolved in 10 mL of anhydrous dichloromethane, and slowly dropped into the reaction system through a constant pressure dropping device. Stir at room temperature for 3-4h. After the completion of the reaction was monitored by TLC, the reaction system was poured into ice water to quench acetyl chloride. Extracted 3 times with 50mL DCM, combined the organic phases, washed the organic phases with 5% sodium hydroxide twice, dried the orga...
Embodiment 2
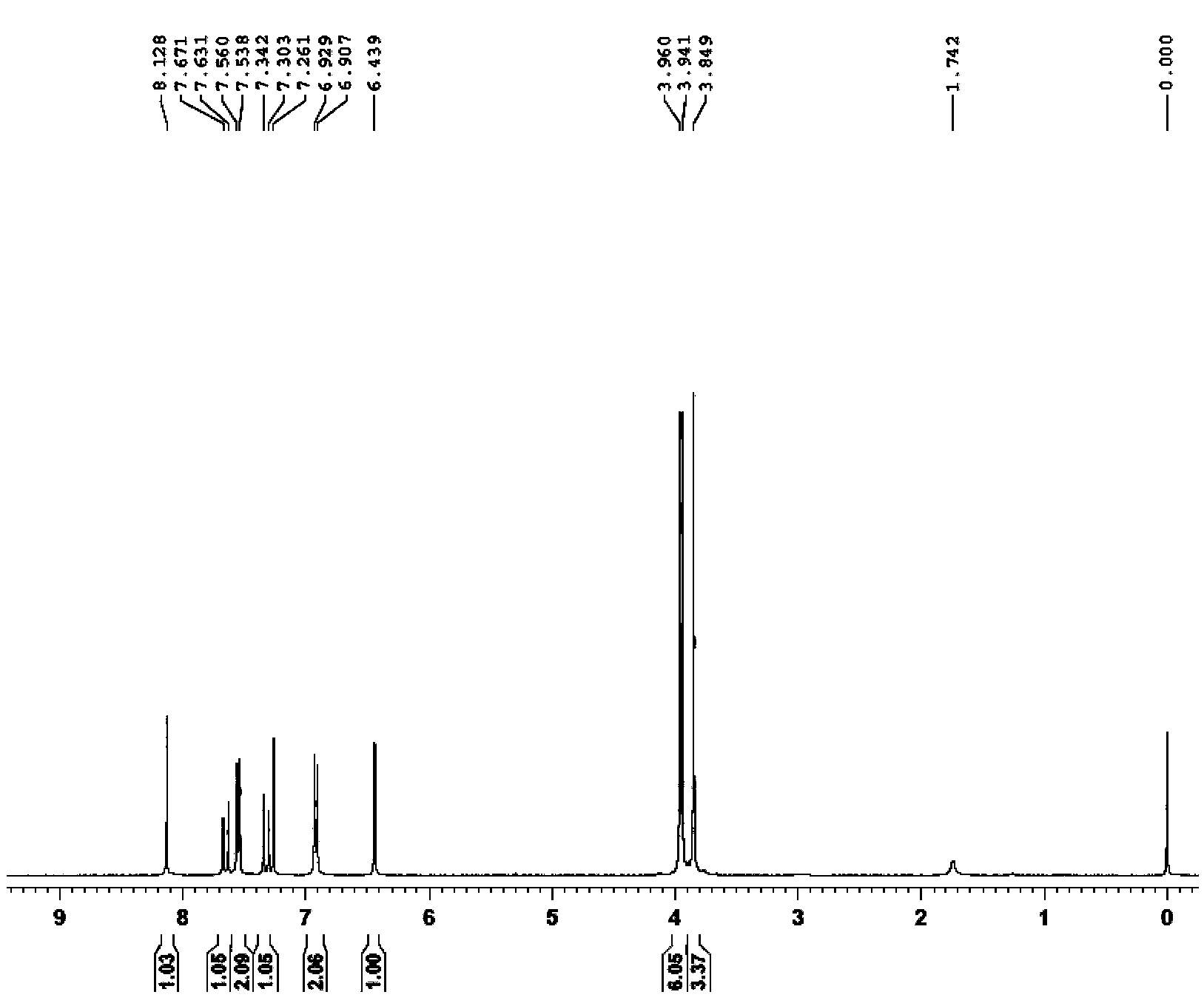
[0050] R is p-fluorophenyl, that is, 4,2',4'-trimethoxy-5'(p-fluorophenyl)chalcone is synthesized as follows:
[0051] 4,2',4'-Trimethoxy-5'iodochalcone (200 mg, 471.44 μmol), dissolved in 1.5 mL dimethylsulfoxide. Add p-fluorophenylboronic acid (79.2mg, 565.73μmol), anhydrous potassium acetate (92.5mg, 942.89μmol), catalyst [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride ( 17mg, 23.57μmol), argon protection. Microwave at 90°C for 1.5h. After the completion of the reaction was monitored by TLC thin layer chromatography, water was added, 20 mL each of ethyl acetate was added, extracted 3 times, the organic phases were combined, dried over anhydrous sodium sulfate, evaporated to dryness, purified by silica gel column chromatography (petroleum ether: ethyl acetate = 8:1) to obtain 110 mg of yellow solid 4,2',4'-trimethoxy-5'(p-fluorophenyl)chalcone, with a yield of 58%.
[0052] 1 HNMR (400MHz, CDCl 3 ):δ / ppm3.85(s,3H),3.90(s,3H),3.99(s,3H),6.57(s,3H),6.92(d,J=8.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com