Ophthalmic medicinal composition containing sirolimus
A composition and drug technology, applied in the directions of drug combinations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve problems such as adverse reactions and unsatisfactory drug effects, and achieve the effect of less adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
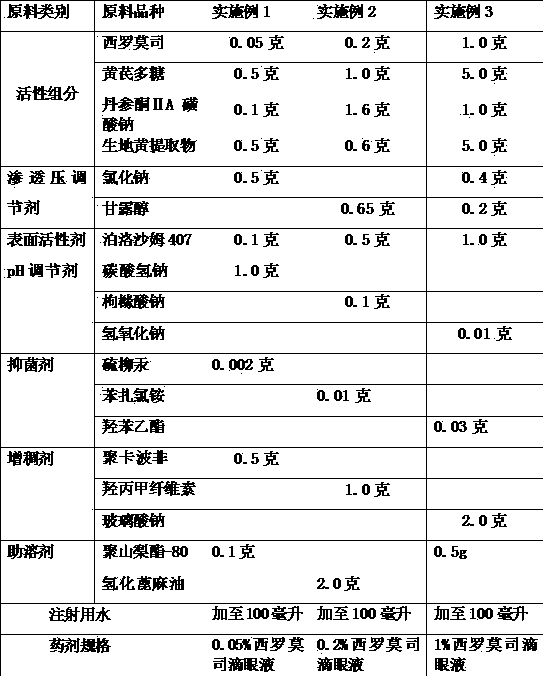
[0024] Example 1-3 Preparation of Compound Sirolimus Eye Drops
[0025] The raw material components and consumptions used for preparing compound sirolimus eye drops are as follows:
[0026]
[0027] Preparation method: take a small amount of water for injection, first add co-solvent, then add sirolimus, astragalus polysaccharide, sodium tanshinone IIA sulfonate and rehmannia glutinosa extract, stir and dissolve into liquid A, set aside; thickener and surfactant Use water for injection to disperse and let it cool down, the liquid is B liquid, and use it for later use; in addition, dissolve the osmotic pressure regulator and bacteriostatic agent with water for injection, stir well and filter to obtain C liquid. Combine the two liquids B and C, then add the dissolved liquid A, add water for injection to the full amount, filter, and pack to obtain. A pH regulator is used to adjust the pH value of the finished eye drops to 5.5-7.5.
Embodiment 4-6
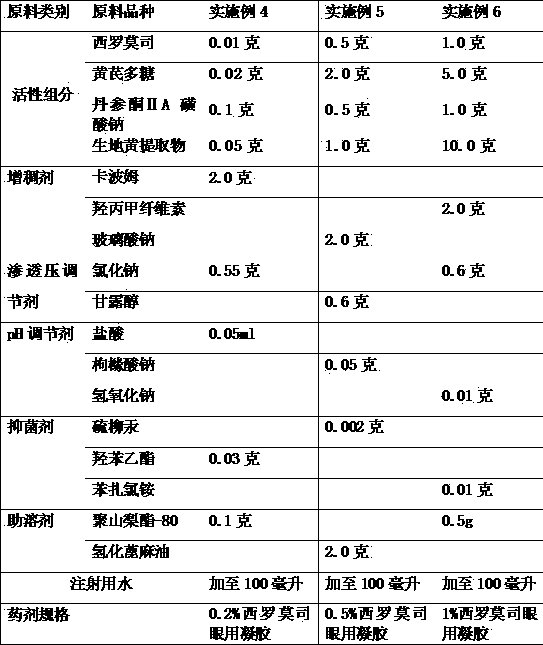
[0028] Example 4-6 Preparation of compound sirolimus ophthalmic gel
[0029] The raw material components and consumptions used for preparing the compound sirolimus ophthalmic gel are as follows:
[0030]
[0031] Preparation method: take a small amount of water for injection, first add co-solvent, then add sirolimus, astragalus polysaccharide, sodium tanshinone IIA sulfonate and rehmannia glutinosa extract, stir and dissolve into liquid A, set aside; thickener and surfactant Use water for injection to disperse and let it cool down, the liquid is B liquid, and use it for later use; in addition, dissolve the osmotic pressure regulator and bacteriostatic agent with water for injection, stir well and filter to obtain C liquid. Combine the two liquids B and C, then add the dissolved liquid A, add water for injection to the full amount, filter, and pack to obtain. A pH regulator is used to adjust the pH value of the finished eye drops to 5.5-7.5.
[0032] Preparation method:...
Embodiment 7-9
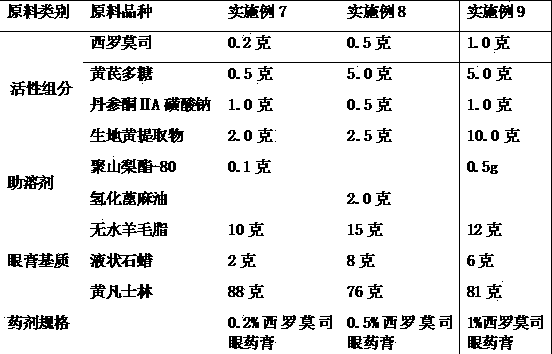
[0034] Example 7-9 Preparation of Compound Sirolimus Ophthalmic Ointment
[0035] The used raw material components and consumption of preparation compound sirolimus eye ointment are as follows:
[0036]
[0037] Preparation method: take a small amount of water for injection, first add co-solvent, add sirolimus, astragalus polysaccharide, sodium tanshinone ⅡA sulfonate and rehmannia glutinosa extract, stir to dissolve, filter aseptically; add appropriate amount of sterilized and cooled liquid paraffin , grind into a fine paste, pass through a 200-mesh sieve, then gradually add sterile and filtered lanolin and yellow petrolatum mixture, mix well, and obtain.
[0038] According to the technical solution of the present invention, the types of excipients that can be used to prepare sirolimus ophthalmic preparations are not limited to those listed in the above table, and the following multiple options can also be selected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com