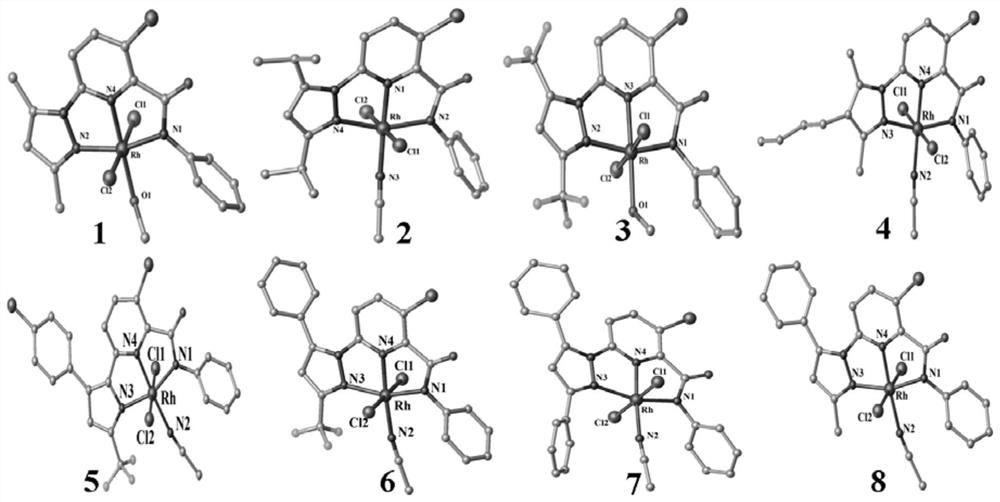
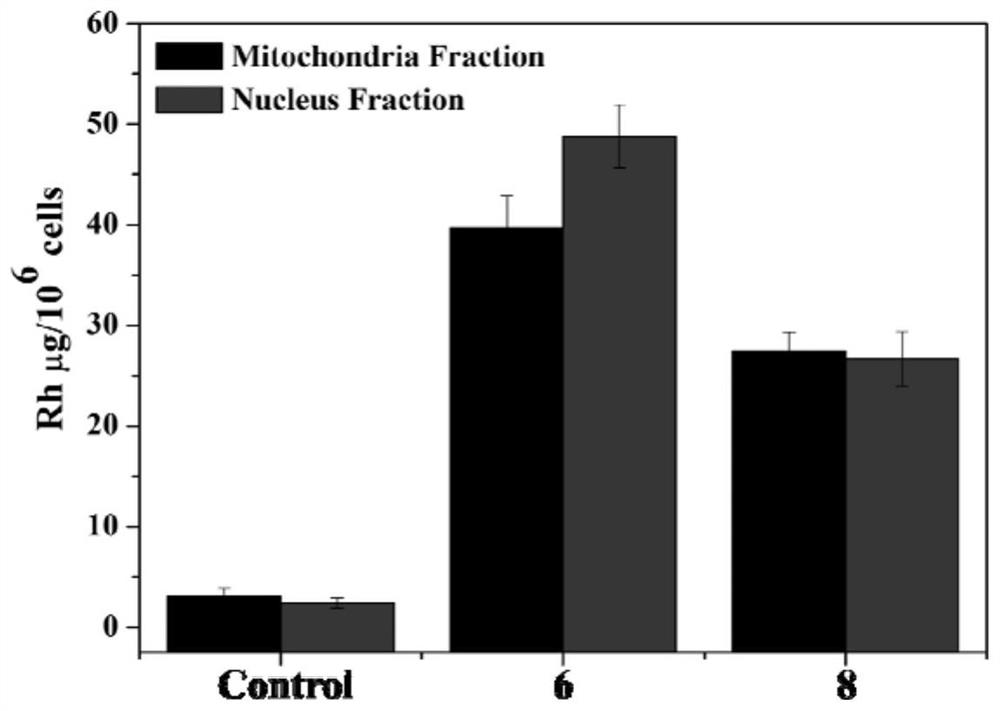
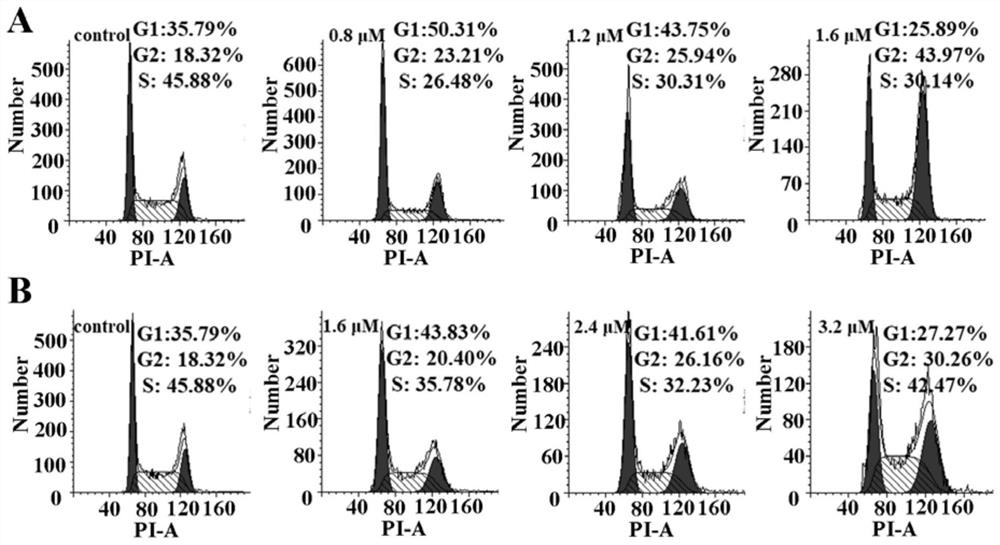
Picolinamide rhodium complex as well as synthesis method and application thereof
The technology of a pyridine amide rhodium and its synthesis method is applied in the field of medicine, which can solve the problems of unseen pyridine amide derivative rhodium complex synthesis and application, unsatisfactory anti-tumor activity, etc., and achieve high yield, easy availability of raw materials, and synthesis simple route effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: Preparation of pyridine amide derivatives (also referred to as ligands in this application) shown in formula (I)
[0058] Prepare according to the following route:
[0059]
[0060] Ia: R 1 = R 2 =CH 3 , R 3 = H;
[0061] Ib:R 1 = R 2 =CH(CH 3 ) 2 , R 3 = H;
[0062] Ic:R 1 = R 2 = CF 3 , R 3 = H;
[0063] Id: R 1 = R 2 =CH 3 , R 3 =(CH 2 ) 3 CH 3 ;
[0064] Ie: R 2 = CF 3 , R 3 = H;
[0065] If: R 2 = CF 3 , R 3 = H;
[0066] Ig: R 3 = H;
[0067] Ih: R 2 =CH 3 , R 3 =H.
[0068] Reaction solvent and condition: i) SOCl 2 , DMF, 85℃, 3h; ii) aniline, CH 3 CN, triethylamine, 80°C, 6h; iii) hydrazine hydrate, ethanol, 80°C; iv) ethanol, 90°C.
[0069] Concrete preparation method is as follows:
[0070] 1) Preparation of compound S2: Add 0.2-0.5 mL of DMF to a mixture of equimolar amounts of 3,6-dichloropicolinic acid (1.9100 g, 10.00 mmol) and thionyl chloride 2 (1.1900 g, 10.00 mmol) Catalyst, heated at 85...
Embodiment 2
[0082] Embodiment 2: the preparation of ligand
[0083] 1. Preparation of ligand Ia: Repeat Example 1, the difference is,
[0084] In step 1), the reaction is carried out at 50°C, and the reaction time is controlled at 6h;
[0085] In step 2), the reaction is carried out at 70° C., the reaction time is controlled at 8 hours, and the amount of triethylamine added is to control the pH of the system=9.5.
[0086] Finally, a white solid powder was obtained.
[0087] Carry out respectively to the powder obtained in this implementation 1 H nuclear magnetic resonance spectrum, elemental analysis and electrospray mass spectrometry and other analyzes confirmed that the obtained powder was ligand Ia.
[0088] ② preparation of ligand Ib: repeat Example 1, the difference is,
[0089] In step 3), the reaction is changed to 70°C, and the reaction time is controlled at 10h;
[0090] In step 4), methanol is used as a solvent, and the reaction is carried out at 95° C. instead.
[0091] F...
Embodiment 3
[0102] Example 3: Complex 1 (i.e. Rh(Ia)Cl 3 ·CH 3 OH) preparation
[0103] Weigh 0.025mmol ligand Ia and 0.056mmol RhCl 3 ·3H 2 O In a 50mL round-bottomed flask, add 8mL of mixed solvent (composed of chloroform and methanol at a volume ratio of 3:1), react at 80°C for 24h, filter the reacted feed liquid, and slowly volatilize the filtrate at room temperature for 3~ After 5 days, reddish-brown lumpy crystals were precipitated in the flask, and the crystals were collected and dried. Yield: 65%.
[0104] The structure was determined by methods such as single crystal X-ray diffraction analysis, and the specific characterization data are as follows:
[0105] HRMS (ESI) C 18 h 18 Cl 3 N 4 o 2 Rh[M-Cl-CH 3 OH] + Theoretical value of m / z: 462.9600; experimental value, 462.9588. Elemental analysis: theoretical value C, 40.67; H, 3.41; N, 10.54. Experimental value C, 40.63; H, 3.43; N, 10.57.
[0106] Select reddish-brown bulk crystals of moderate size and place them on an ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com