Binuclear copper complex with 9-formyl-10-benzothiazolyl anthracene as a ligand, its synthesis method and application
A synthetic method and compound technology, applied in the field of medicine, can solve problems such as significant toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Preparation of the compound shown in formula (II) (i.e. 9-formyl-10-benzothianthracene, hereinafter also referred to as ligand)
[0029] 1) Dissolve 5.0mmol of 9,10-anthracenedicarbaldehyde and 6.5mmol of 2-hydrazinobenzothianthracene hydrazone in 100mL of ethanol, reflux reaction at 78°C, and track and detect with TLC until the reaction is complete (about 6h ), the reaction was stopped, and the reaction solution was filtered while it was hot, and the resulting solid was vacuum-dried for 10 h to obtain a mixed product with a yield of 85%;
[0030] 2) Put the resulting mixed product on silica gel column chromatography, elute with a mixed eluent composed of petroleum ether and dichloromethane (volume ratio of petroleum ether and dichloromethane is 1:3), and simultaneously use thin layer chromatography Follow up and monitor, collect the eluate containing the target product, concentrate and dry it to obtain a reddish-brown solid product with a yield of 65%.
...
Embodiment 2
[0038] Embodiment 2: the preparation of compound shown in formula (II)
[0039] Example 1 was repeated, except that in step 2), a mixed eluent composed of petroleum ether and dichloromethane in a volume ratio of 1:7 was used for elution.
[0040] As a result, a reddish-brown solid product was obtained with a yield of 53%.
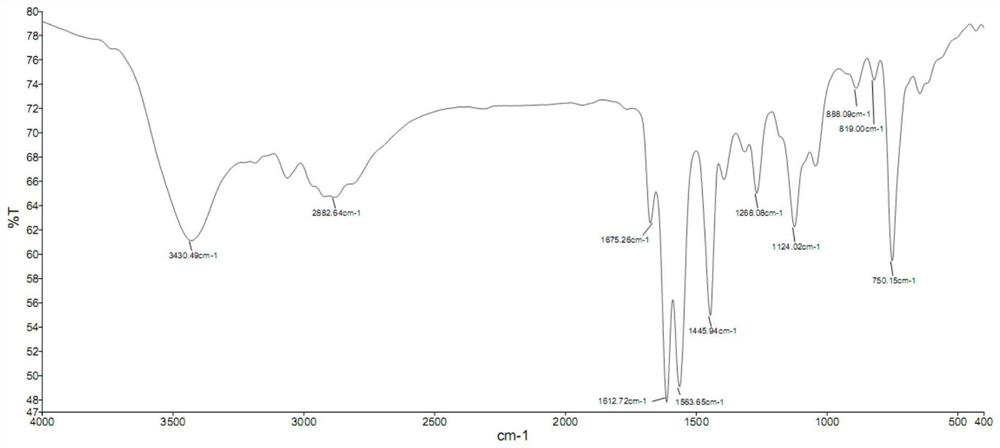
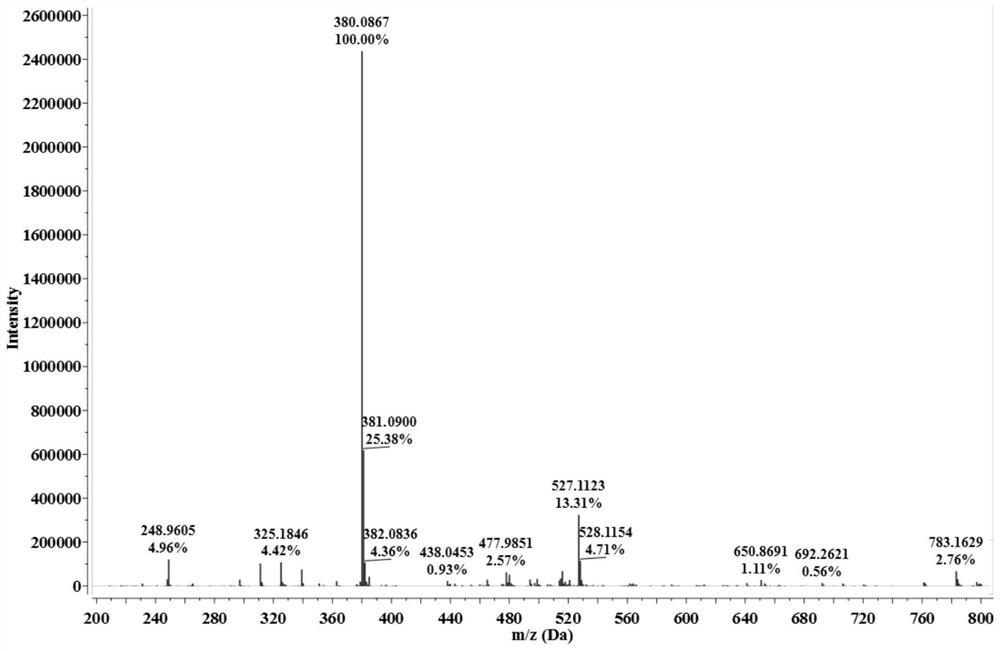
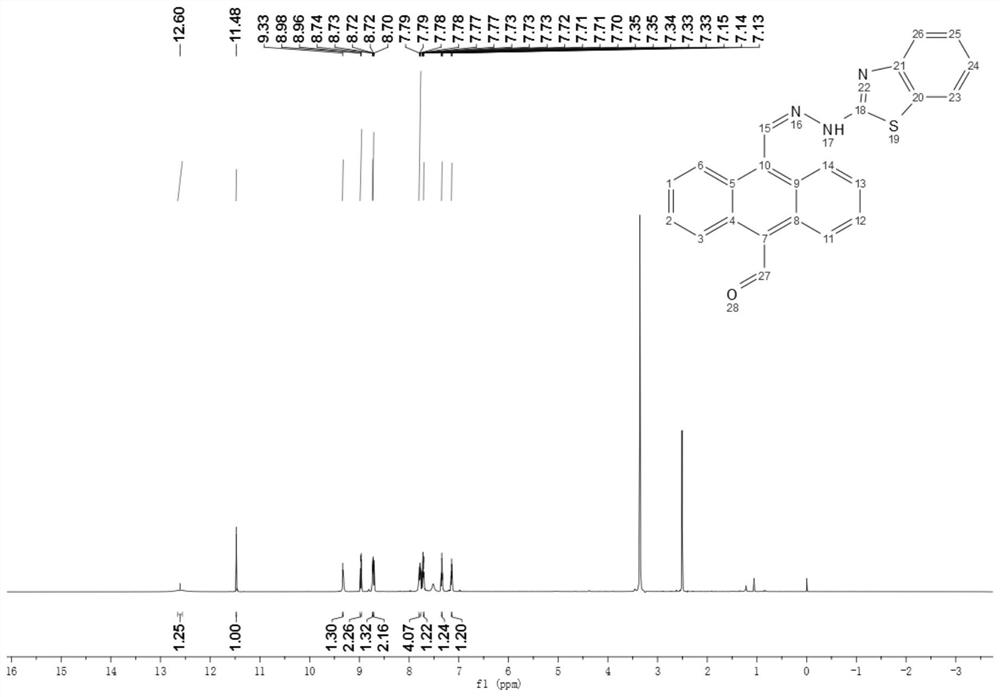
[0041] The reddish-brown solid product obtained in this embodiment was subjected to infrared spectrum, electrospray mass spectrum, 1 H NMR spectrum and 13 C nuclear magnetic resonance spectrum identification, determined to be the compound shown in formula (II).
Embodiment 3
[0042] Embodiment 3: the preparation of compound shown in formula (II)
[0043] Repeat Example 1, the difference is:
[0044] In step 1), ethanol is replaced with methanol, and the reaction is carried out at 50° C.;
[0045] In step 2), a mixed eluent composed of petroleum ether and dichloromethane in a volume ratio of 1:1 was used for elution.
[0046] As a result, a reddish-brown solid product was obtained with a yield of 36%.
[0047] The reddish-brown solid product obtained in this embodiment was subjected to infrared spectrum, electrospray mass spectrum, 1 H NMR spectrum and 13 C nuclear magnetic resonance spectrum identification, determined to be the compound shown in formula (II).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com