Two rare earth coordination compounds constructed on the basis of 2-methyl-5,7-dibromo-8-hydroxyquinoline, preparation method and applications thereof,
A technology of rare earth complexes and hydroxyquinoline, applied in medical preparations containing active ingredients, pharmaceutical formulas, organic chemical methods, etc., can solve the problems of no anti-proliferation activity and achieve significant anti-proliferation effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
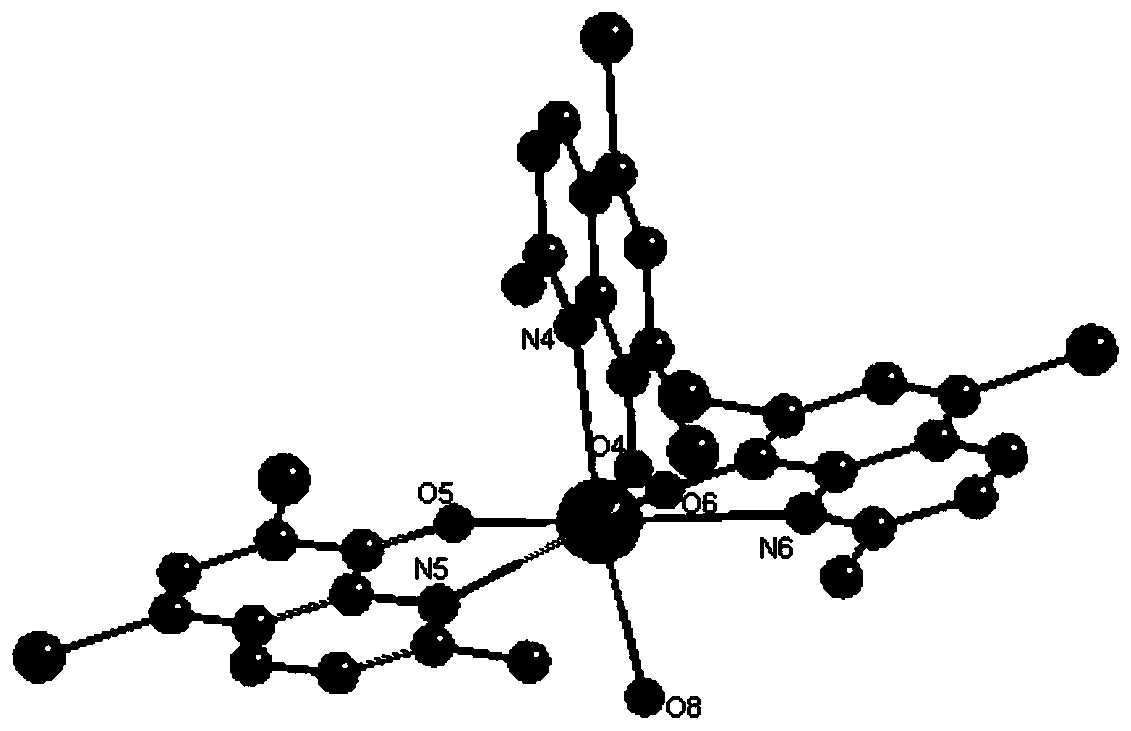
[0030] Example 1: Complex 1 (ie complex [Sm(C 10 H 6 NOBr 2 ) 3 (H 2 O)]) Preparation
[0031] Add 0.1mmol (44mg) Sm(NO 3 ) 3 ·6H 2 O and 0.2mmol (64mg) of ligand 2-methyl-5,7-dibromo-8-hydroxyquinoline (also referred to as ligand HL in this application) were added to a Pyrex tube with a closed end of about 18 cm in length, and added 1.25mL by CHCl 3 And H 2 O composition of mixed solvent (CHCl 3 And H 2 The volume ratio of O is 4:1), add 2 drops of Et 3 N (the pH value of the system after stirring is 7.5), the Pyrex tube is evacuated and the other end is sealed. Put the sealed Pyrex tube into an oven at 80°C, react for 72 hours, take it out, and slowly cool to room temperature. It can be observed that yellow strips of crystals are deposited at the bottom of the Pyrex tube. Collect the crystals and dry. The yield was 68% (based on Sm(NO 3 ) 3 ·6H 2 O).
[0032] Characterize the product obtained in this example:
[0033] 1) Elemental analysis (%): experimental value: C, 32.98, H, 1....
Embodiment 2
[0056] Example 2: Preparation of Complex 1
[0057] Repeat Example 1, the difference is: the mixed solvent CHCl 3 And H 2 Change the volume ratio of O to 1:1, use Et 3 N adjusts the pH of the system to 8.0. The reaction is changed to 60°C and the reaction time is 60h.
[0058] As a result, yellow bar-shaped crystals were obtained. The yield is 67% (based on Sm(NO 3 ) 3 ·6H 2 O).
[0059] The product obtained in this example was subjected to elemental analysis, infrared analysis and single crystal diffraction analysis, and it was determined that the obtained yellow stripe crystals were the target product complex 1.
Embodiment 3
[0060] Example 3: Preparation of Complex 1
[0061] Repeat Example 1, the difference is: the mixed solvent CHCl 3 And H 2 Change the volume ratio of O to 2:1, use Et 3 N adjusts the pH of the system to 6.5, and the reaction is carried out at 100°C.
[0062] As a result, yellow bar-shaped crystals were obtained. Yield 63% (based on Sm(NO 3 ) 3 ·6H 2 O).
[0063] The product obtained in this example was subjected to elemental analysis, infrared analysis and single crystal diffraction analysis, and it was determined that the obtained yellow stripe crystals were the target product complex 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com