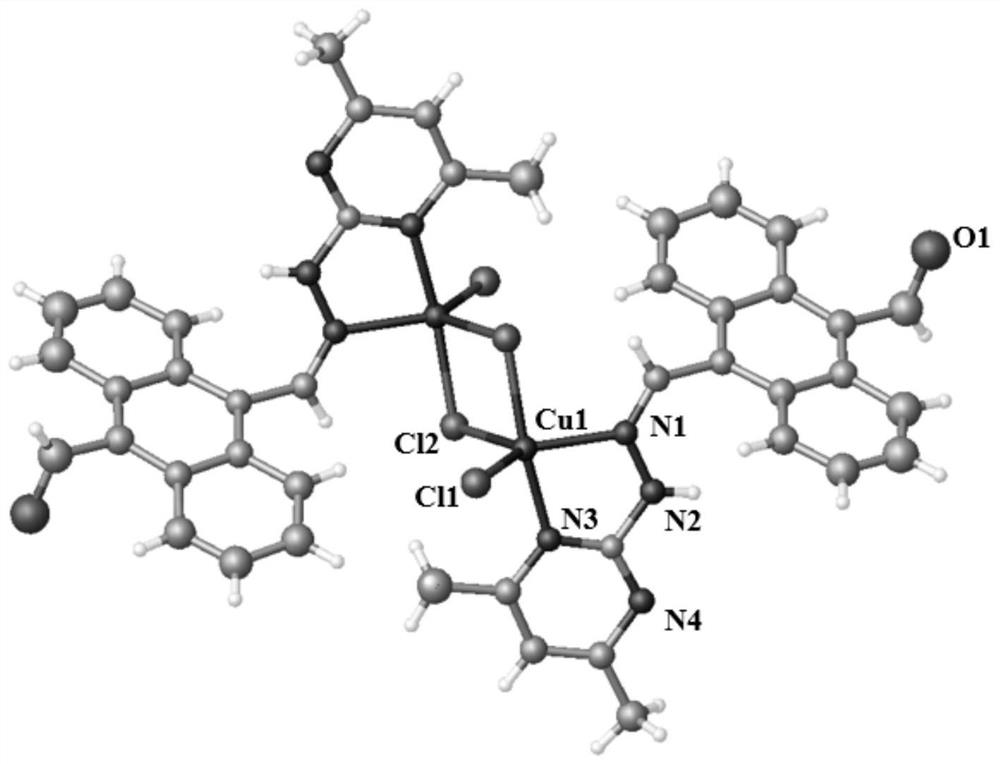
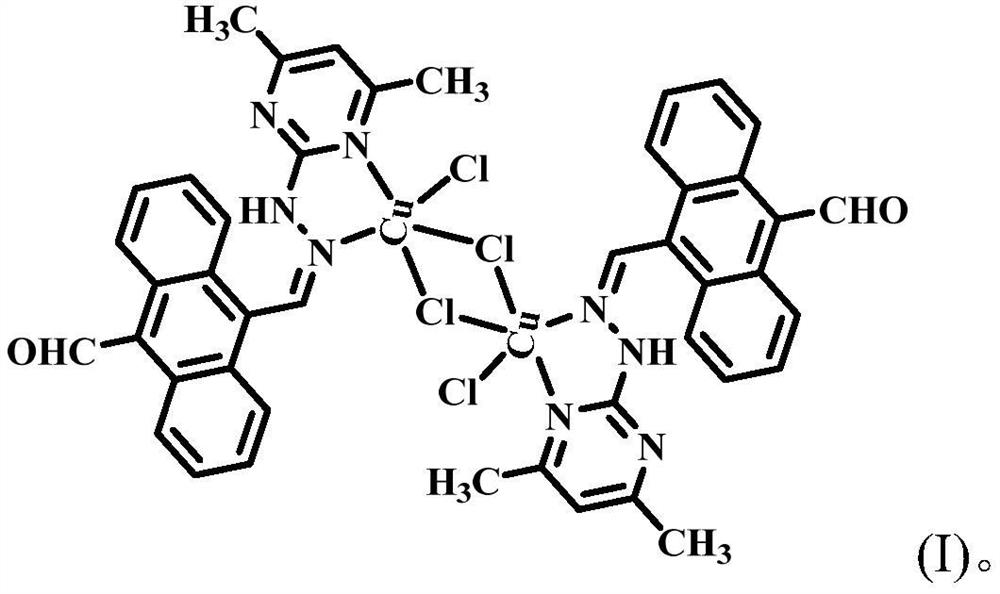
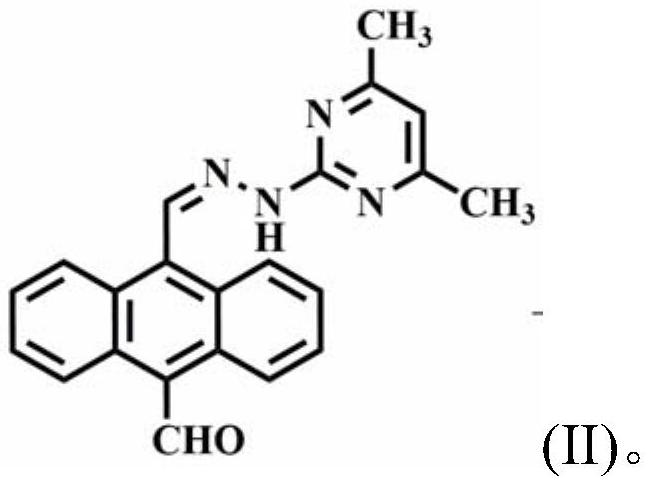
Copper(ii) complexes using 9-aldehyde-10-pyrimanthracene hydrazone as ligands and their synthesis and application
A synthetic method and compound technology, applied in the field of medicine, can solve problems such as insignificant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]Example 1: Preparation of the compound (i.e., 9-aldehyde-10- (4 ', 6'-dimethylpyrimidine) 腙, hereinafter referred to as the ligand)
[0022]1) Take 5.0 mmol of 9,10-anthrafaldehyde and 6.5 mmol of 2-hydrazine-4,6-dimethylpyrimidine dissolved in 100 mL of methanol, and refluxed under 65 ° C and followed by TLC tracking. Complete (approximately 8 h), stopped the reaction, and the reaction liquid was filtered, the resulting solid was dried in vacuo to dry 10 h, resulting in a mixed product, yield 85%;
[0023]2) Elution of the resulting mixed product by silica gel column, mixed cleansing released by petroleum ether and dichloromethane eluting eluting eluting eluting, at the same time Monitoring, the eluent containing the target product was collected, concentrated, dried to give a yellow solid product, and the yield was 65%.
[0024]Infrared spectroscopy, elemental analysis, electrospray mass spectrometry, respectively, the yellow solid product obtained in this example,1H Nuclear magnetic ...
Embodiment 2
[0032]Example 2: Preparation of the compound of formula (II)
[0033]1) Take 5.0 mmol of 9,10-anthrafaldehyde and 5.0 mmol of 2-hydrazine-4,6-dimethylpyrimidine dissolved in 100 mL of methanol, and reflow under 65 ° C, followed by TLC tracking detection to the reaction Complete (approximately 6 h), the reaction was stopped, and the reaction liquid was filtered, the resulting solid was dried in vacuo to dry 10 h, resulting in a mixed product, yield 75%;
[0034]2) Elution of the resulting mixed product by silica gel column, mixed washing removal (petroleum ether, and dichloromethane) eluted, and trace at the same time Monitoring, the eluent containing the target product was collected, concentrated, dried to give a yellow solid product, and the yield was 45%.
[0035]Infrared spectroscopy, elemental analysis, electrospray mass spectrometry, respectively, the yellow solid product obtained in this example,1H Nuclear magnetic resonance spectrum and13C N-N-magnetic resonance spectrum identificatio...
Embodiment 3
[0036]Example 3: Preparation of the compound of formula (II)
[0037]1) Take 5.0 mmol of 9,10-anthraflicaldehyde and 7.0 mmol of 2-hydrazine-4,6-dimethylpyrimidine in 100 ml of ethanol, and reflow under conditions of 78 ° C, and track the reaction with TLC Complete (approximately 7 hours), the reaction was stopped, and the reaction liquid was filtered, the resulting solid was dried in vacuo to dry the mixed product, and the yield was 65%;
[0038]2) Elution of the resulting mixed product by silica gel column, mixed washing removal (petroleum ether, and dichloromethane) eluted by petroleum ether and dichloromethane, while taking a thin layer chromatography Monitoring, eluent containing the target product was collected, concentrated, dried to give a yellow solid product, 50% yield.
[0039]Infrared spectroscopy, elemental analysis, electrospray mass spectrometry, respectively, the yellow solid product obtained in this example,1H Nuclear magnetic resonance spectrum and13C N-N-magnetic resonance...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com