Palonosetron preparation for injection and preparation method thereof
A technology for palonosetron and injection, which is applied in the field of medicine, can solve problems such as general solubility, impact on safety, and impact on convenience, and achieve the effects of good reconstitution, smooth surface, and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
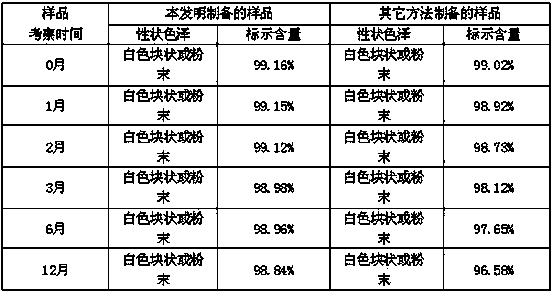
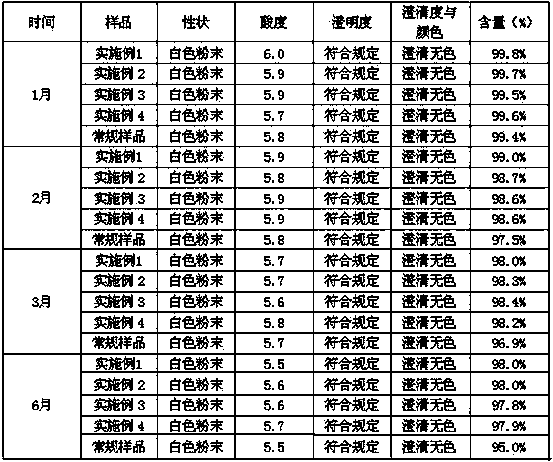
Embodiment 1
[0021] The formula of the described palonosetron hydrochloride freeze-dried preparation of every 1000 bottles consists of:
[0022] Palonosetron Hydrochloride 0.25g
[0023] β-cyclodextrin 10g
[0024] Mannitol 20g
[0025] Water for injection 1L
[0026] Preparation process: 1. Weigh palonosetron hydrochloride and β-cyclodextrin, add 80% water for injection and stir to dissolve, then add mannitol, stir to dissolve completely, and use citrate-disodium phosphate buffer solution (pH4.0) adjust the pH value to 4.0-6.0, add water for injection to the full amount, add a needle with a total volume of 0.05-0.1% (g / ml), stir and absorb with carbon for 20 minutes, and decarburize by filtration.
[0027] 2. Fine filter with 0.22μm microporous membrane.
[0028] 3. Inspection of semi-finished products.
[0029] 4. Filling, press half stopper.
[0030] 5. Freeze-drying: first cool down the freeze-drying box to -40°C ~ -50°C, then put the sample into it to make it freeze quickly, the...
Embodiment 2
[0032] The formula of the described palonosetron hydrochloride freeze-dried preparation of every 1000 bottles consists of:
[0033] Palonosetron Hydrochloride 0.25g
[0034] Hydroxypropyl-β-cyclodextrin 15g
[0035] Mannitol 18g
[0036] Water for injection 1L
[0037] Preparation process: 1. Weigh palonosetron hydrochloride and hydroxypropyl-β-cyclodextrin, add 80% water for injection and stir to dissolve, then add mannitol, stir to dissolve completely, and use citric acid-phosphoric acid Disodium buffer solution (pH 4.0) was used to adjust the pH value to 4.0-6.0, add water for injection to the full amount, add needles with a total volume of 0.05-0.1% (g / ml), stir and adsorb with carbon for 20 minutes, and decarburize by filtration.
[0038] 2. Fine filter with 0.22μm microporous membrane.
[0039] 3. Inspection of semi-finished products.
[0040] 4. Filling, press half stopper.
[0041] 5. Freeze-drying: first cool down the freeze-drying box to -40°C~-50°C, then put in ...
Embodiment 3
[0043] The formula of the described palonosetron hydrochloride freeze-dried preparation of every 1000 bottles consists of:
[0044] Palonosetron Hydrochloride 0.5g
[0045] β-cyclodextrin 20g
[0046] Mannitol 25g
[0047] Water for injection 2L
[0048] Preparation process: 1. Weigh palonosetron hydrochloride and β-cyclodextrin, add 80% water for injection and stir to dissolve, then add mannitol, stir to dissolve completely, and use citrate-disodium phosphate buffer solution (pH 4.0) adjust the pH value to 4.0-6.0, add water for injection to the full amount, add a needle with a total volume of 0.05-0.1% (g / ml), stir and adsorb with carbon for 20 minutes, and decarburize by filtration.
[0049] 2. Fine filter with 0.22μm microporous membrane.
[0050] 3. Inspection of semi-finished products.
[0051] 4. Filling, press half stopper.
[0052] 5. Freeze-drying: first cool down the freeze-drying box to -40°C~-50°C, then put in the sample to make it freeze quickly, the time i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com