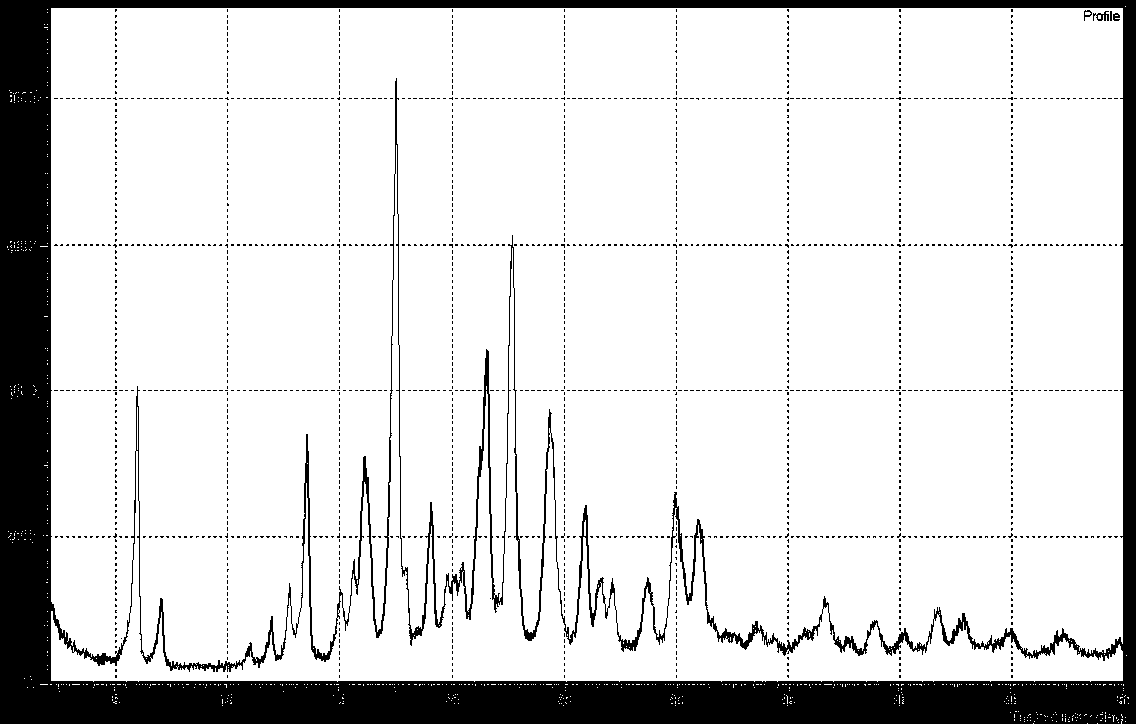
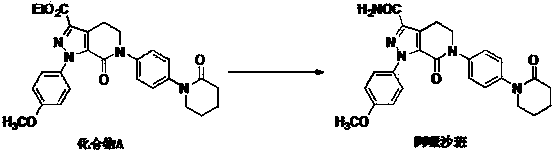
Crystal form B of Apixaban and preparation method thereof
A technology of apixaban and crystal form, applied in the direction of organic chemistry and the like, can solve the problems of complex preparation process of apixaban, high equipment requirements, great influence on preparation quality, etc., and achieves easy industrial production, simple preparation method, The effect of facilitating the transformation of crystal forms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Put compound A (10g) into a 1L reaction flask, add chloroform (600ml) and formamide (100g), stir and heat to reflux, add trimethyl orthoformate (1.5g) and trifluoroacetic acid (0.5g), The mixture was stirred for an additional 30 minutes, cooling below 50°C. A methanol solution (9 g) of sodium methoxide was added, and the reaction was determined to be complete by HPLC. Then, 200 ml of water was added, and the mixture was stirred and separated. The organic layer was concentrated under reduced pressure, 70% of the solvent was removed, cooled to 0°C for crystallization, suction filtered, and dried to obtain 7.3 g of apixaban B crystal form product, with a yield of 77.6%.
Embodiment 2
[0037] Put compound A (5g) into a 1L reaction flask, add toluene (600ml) and formamide (100g), stir and heat to reflux, add trimethyl orthoformate (1.5g) and trifluoroacetic acid (0.5g), the The mixture was stirred for an additional 30 minutes, dropping below 50°C. A methanol solution (5 g) of sodium methoxide was added, and the reaction was determined to be complete by HPLC, and 200 ml of water was added, and the mixture was stirred and separated. The organic layer was concentrated under reduced pressure, 70% of the solvent was removed, cooled to 0°C for crystallization, suction filtered, and dried to obtain 3.5 g of apixaban B crystal form product, with a yield of 74.4%.
Embodiment 3
[0039] Put compound A (10g) into a 1L reaction flask, add tetrahydrofuran (300ml), ethyl acetate (300ml) and formamide (100g), stir and heat to reflux, add trimethyl orthoformate (1.5g) and trifluoroacetic acid (0.5 g), the mixture was stirred for an additional 30 minutes, cooling to below 50°C. A methanol solution (10 g) of sodium methoxide was added, and the reaction was confirmed to be complete by HPLC. Then, 200 ml of water was added, and the mixture was stirred and separated. The organic layer was concentrated under reduced pressure, 70% of the solvent was removed, cooled to 0°C for crystallization, suction filtered, and dried to obtain 6.3 g of apixaban B crystal form product, with a yield of 67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com