Method for preparing titanium dioxide nanoparticles
A nanoparticle, titanium dioxide technology, applied in titanium dioxide, titanium oxide/hydroxide, nanotechnology and other directions, to achieve the effect of simple and easy process, reduced production cost and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The total amount is 180ml, and the volume ratio of cyclohexane:ethanol=1:0.5 is mixed as a solvent, and 36ml of tetrabutyl titanate is added to the mixed solvent. After stirring evenly, add 8ml of concentrated hydrochloric acid (36-38%) according to the ratio of tetrabutyl titanate:concentrated hydrochloric acid=1:0.22 by volume. Reflux for 10 hours after heating up to boiling. The reflux reaction product (light yellow transparent liquid) was added dropwise with 3 times the volume of ethanol under the condition of stirring, the stirring was stopped, and it settled overnight. The resulting product was washed, centrifuged and dried.
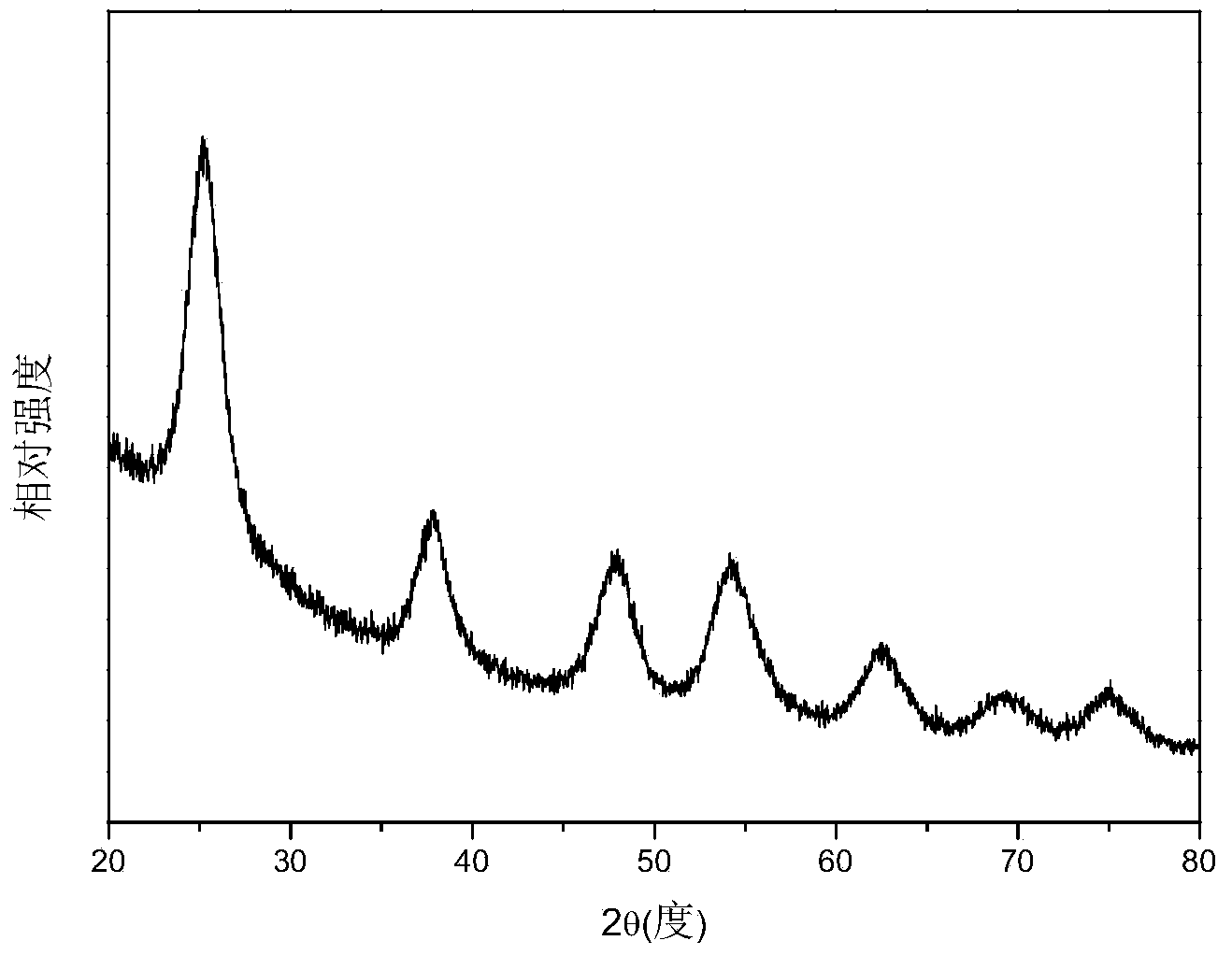
[0033] Figure 1~3 The X-ray diffraction spectrum, transmission electron microscope pictures and high resolution transmission electron microscope pictures of the prepared titanium dioxide nanoparticles are given respectively. The obtained product is monodisperse anatase phase titanium dioxide nanoparticles with a particle diameter of less...
Embodiment 2
[0035] The total amount is 180ml, and the volume ratio of cyclohexane:ethanol=1:5 is mixed as a solvent, and 36ml of tetrabutyl titanate is added to the mixed solvent. After stirring evenly, add 8ml of concentrated hydrochloric acid (36-38%) according to the ratio of tetrabutyl titanate:concentrated hydrochloric acid=1:0.22 by volume. Reflux for 10 hours after heating up to boiling. The reflux reaction product (light yellow transparent liquid) was added dropwise with 3 times the volume of ethanol under the condition of stirring, the stirring was stopped, and it settled overnight. The resulting product was washed, centrifuged and dried.
[0036] Compared with Example 1, the volume ratio of the reaction solvent cyclohexane:ethanol was changed to 1:5, and the obtained product was titanium dioxide nanoparticles in anatase phase with no obvious change in morphology. Figure 4 X-ray diffraction pattern for the product. When the volume ratio of the reaction solvent hexamethylene: ...
Embodiment 3
[0038] The total amount is 180ml, and the volume ratio of cyclohexane:ethanol=1:0.5 is mixed as a solvent, and 36ml of tetrabutyl titanate is added to the mixed solvent. After stirring evenly, add 9 ml of concentrated hydrochloric acid (36-38%) according to the ratio of tetrabutyl titanate:concentrated hydrochloric acid=1:0.25 by volume. Reflux for 10 hours after heating up to boiling. The reflux reaction product (light yellow transparent liquid) was added dropwise with 3 times the volume of ethanol under the condition of stirring, the stirring was stopped, and it settled overnight. The resulting product was washed, centrifuged and dried.
[0039]Compared with Example 1, the reactant volume ratio of tetrabutyl titanate:concentrated hydrochloric acid was changed to 1:0.25, and the obtained product was titanium dioxide nanoparticles in anatase phase with no obvious change in morphology. Figure 5 X-ray diffraction pattern for the product. When reactant volume ratio tetrabutyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com