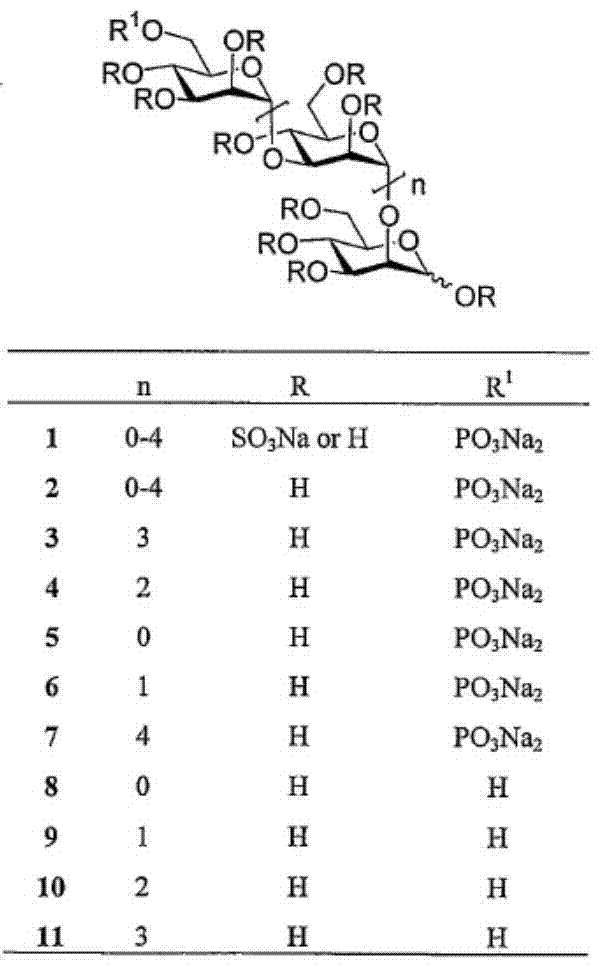
Sulfated oligosaccharide derivatives
A kind of derivative and compound technology, applied in the field of sulfated oligosaccharide derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1: from Pichia total synthesis neutral manna-oligosaccharide (8-11)
[0086]
[0087] Benzyl 2-O-(3-O-allyl-2,4,6-tri-O-benzoyl-α-D-mannopyranosyl)-3,4,6-tri-O- Benzyl-α-D-mannopyranoside (24)
[0088] 3-O-allyl-2,4,6-tri-O-benzoyl-α-D-mannopyranosyl trichloroimidate [26] (902mg, 1.21mmol) and benzyl A mixture of 3,4,6-tri-O-benzyl-α-D-mannopyranoside [27] (723mg, 1.34mmol) in 1,2-DCE (10mL) on molecular sieves (1.0g of powder) under argon atmosphere (30 min). The mixture was cooled (0° C.) with continued stirring (10 minutes) before adding TMSOTf (219 μL, 1.21 mmol). After a period of time (10 minutes), Et was introduced 3N (100 μL) and the mixture was filtered. The solvent was evaporated and the residue was subjected to FC (10-50% EtOAc / hexanes) to give the tribenzoate (24) (1.14 g, 84%) as a colorless oil. 1 H NMR (CDCl 3 )δ3.67-3.81,3.88-3.95,4.06-4.15,4.30-4.35(4m,12H;H-2 I ,-3 I ,-4 I ,-5 I ,-6a I ,-6b I ,-3 II ,-5 II ,-6a II ,-6b I...
Embodiment 2
[0110] Embodiment 2: benzyl glycoside polysulfate (PG 500)
[0111]
[0112] Peracetate 12
[0113] The pentasaccharide 11 (1.03 g, 95% M5), sodium acetate (1.2 g) and acetic anhydride (50 mL) were heated under a drying tube at 140 °C overnight with stirring. The mixture was cooled to room temperature, evaporated to dryness, taken up in EtOAc, washed with brine (x3) and flash chromatographed (40 g silica gel, 80:20 EtOAc:Hx) to give a glassy 810mg peracetate 12. 1 H NMR (400MHz, CDCl 3 )δ6.14(d,0.84H,J=2.0,αHl I ),5.71(d,0.16H,J=0.9,βHl I ), 5.30-5.10 (m, 8H), 5.00-4.85 (m, 7H), 4.25-3.70 (m, 19H), 2.20-1.90 (m, 51H). for C 64 h 87 o 43 Calculated value of HRMS [M+H] + 1543.4623, measured value 1543.4599.
[0114] General method for direct saccharification of peracetylated oligosaccharides:
[0115] The alcohol (6 equiv) was added to the peracetate (eg, 12) (1 equiv) in MS in dry DCM (0.03M). In some cases, adding a small amount MS powder. Boron trifluoride e...
Embodiment 3
[0122] Embodiment 3: Octyl glucoside polysulfate (PG 501)
[0123]
[0124] Octyl Glycoside 14
[0125] The saccharification using 12 and octanol afforded the product (14) as a colorless gum, 207 mg, 66% (Rf = 0.41, hexane-EtOAc = 1:3). 1 H NMR (CDC1 3 ,400MHz)δ5.23-5.09(m,8H),4.96-4.82(m,8H),4.23-3.71(m,19H),3.59(dt,1H,J=9.4,6.8,OCH 2 R),3.35(dt,1H,J=9.4,6.8,OCH 2 R), 2.11, 2.10(2), 2.09(8), 2.06, 2.05, 2.04(4), 2.04(1), 2.03(8), 2.03, 2.02, 2.01, 1.99(3), 1.98(8), 1.96, 1.94 and 1.90 (16s, 48H, 16×Ac), 1.52 (quintet, 2H, J=7.2, CH 2 ),1.27-1.18(m,10H,(CH 2 ) 5 ),0.80(t,3H,J=7.2,CH 3 ); 13 C NMR (CDCl 3 ,100MHz)δ170.4(0)(2C),170.3(8)(2C),170.3,170.2,170.1,169.9(2C),169.8(2),169.7(5),169.6,169.5,169.4(4) ,169.3(5),169.3(16×CO,3 overlapping),99.1(2C),98.8,98.7,98.0(5×sugar-Cl),77.0,75.0,74.8(3),74.7(5),71.0 ,70.8,70.7,70.1,69.4(9),69.4(7),69.3(0),69.2(7),69.2,68.3,68.2(0),68.1(6),67.2,66.6(4),66.6( 0), 66.1, 65.4, 62.4, 62.3, 61.8 and 61.5 (25C, the sugar carbon ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com