Method for preparing manganese tungstate nano-sheets by molten salt method
A technology of manganese tungstate nanometer and molten salt method, which is applied in nanotechnology, chemical instruments and methods, manganese compounds, etc., to achieve the effects of no environmental pollution, simple equipment, and easy control of conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
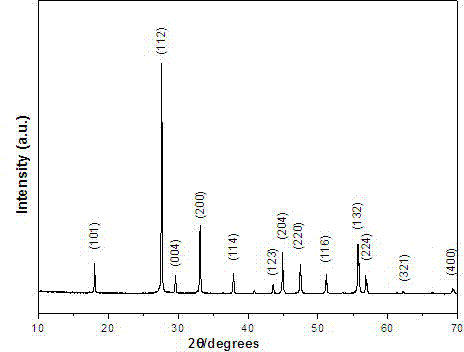
Image
Examples
Embodiment 1
[0024] Step 1. Make sodium tungstate into a solution and adjust the WO in the solution 4 2- The ion concentration is 0.5mol / L;
[0025] Step 2, manganese chloride is made into solution, adjusts the Mn in the solution 2+ The ion concentration is 0.5mol / L;
[0026] Step 3. Mix the sodium tungstate aqueous solution prepared in step 1 with the manganese chloride aqueous solution prepared in step 2, wherein the molar ratio of sodium tungstate and manganese chloride is 1:1, and stir the obtained precipitation solution for 10 minutes Afterwards, suction filtration is carried out, and the precursor is obtained after drying;
[0027] Step 4, fully grind the precursor and lithium nitrate obtained in step 3, wherein the weight ratio of the precursor to lithium nitrate is 1:3, put the mixture in a corundum crucible, then put the crucible into an electric furnace, and heat After keeping warm at ℃ for 5 hours, take it out and cool it down;
[0028] Step 5. Finally, the cooled sample wa...
Embodiment 2
[0031] Step 1. Make sodium tungstate into a solution and adjust the WO in the solution 4 2- The ion concentration is 1.2mol / L;
[0032] Step 2, manganese chloride is made into solution, adjusts the Mn in the solution 2+ The ion concentration is 1.2mol / L;
[0033] Step 3. Mix the sodium tungstate aqueous solution prepared in step 1 with the manganese chloride aqueous solution prepared in step 2, wherein the molar ratio of sodium tungstate and manganese chloride is 1:1, and stir the obtained precipitation solution for 20 minutes Afterwards, suction filtration is carried out, and the precursor is obtained after drying;
[0034] Step 4, fully grind the precursor and lithium nitrate obtained in step 3, wherein the weight ratio of the precursor to lithium nitrate is 1:6, put the mixture in a corundum crucible, then put the crucible into an electric furnace, and heat After keeping warm at ℃ for 3 hours, take it out and cool it down;
[0035] Step 5. Finally, the cooled sample wa...
Embodiment 3
[0037] Step 1. Make sodium tungstate into a solution and adjust the WO in the solution 4 2- The ion concentration is 2.0mol / L;
[0038] Step 2, manganese chloride is made into solution, adjusts the Mn in the solution 2+ The ion concentration is 2.0mol / L;
[0039] Step 3. Mix the sodium tungstate aqueous solution prepared in step 1 with the manganese chloride aqueous solution prepared in step 2, wherein the molar ratio of sodium tungstate and manganese chloride is 1:1, and stir the obtained precipitation solution for 30 minutes Afterwards, suction filtration is carried out, and the precursor is obtained after drying;
[0040] Step 4. Fully grind the precursor and lithium nitrate obtained in step 3. The weight ratio of the precursor to lithium nitrate is 1:10. After keeping warm at ℃ for 2 hours, take it out and cool it down;
[0041] Step 5. Finally, the cooled sample was washed with deionized water and then dried to obtain well-crystallized manganese tungstate nanosheets....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com