Preparation method and application of graphite type carbon nitride nano-rod modified electrode
A technology of graphite-type carbon nitride and modified electrodes, applied in the direction of material electrochemical variables, etc., to achieve high detection sensitivity, control of heavy metal pollution, and wide practical application space
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
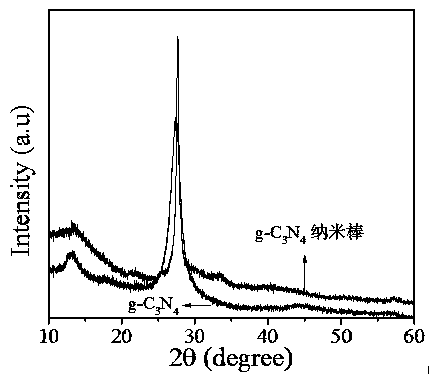
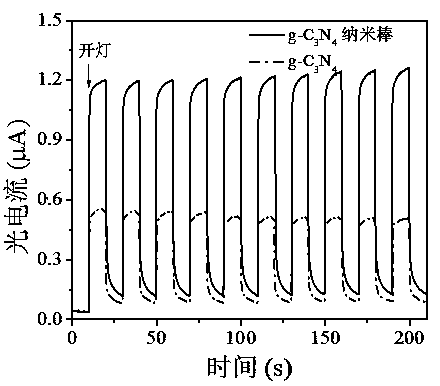
Image
Examples
Embodiment 1
[0031] (1) Put 2 g of dicyandiamide into a semi-closed alumina crucible, raise the temperature to 350 oC at a rate of 2.9 oC per minute under nitrogen atmosphere, keep at this temperature for 2 h, and then The heating rate was 3.3 oC, the temperature was raised to 540 oC, kept at this temperature for 2 h, and then dropped to room temperature naturally. The obtained product was washed four times with deionized water and absolute ethanol, and dried at 50 oC for 12 h to obtain graphite carbon nitride as a yellow solid powder.
[0032] (2) Add 140 mg NH 4 Dissolve Cl into 20 mL deionized water to form NH 4 Cl solution. Immediately followed by 40 mg g-C 3 N 4 Dispersed to the above NH 4 Cl solution, stirred for 30 min, and then sonicated for 30 min to form g-C 3 N 4 Dispersions. The above suspension was transferred to a polytetrafluoroethylene-lined reactor, reacted at 160 °C for 12 h, and then cooled to room temperature naturally. The final product was washed 4 times wit...
Embodiment 2
[0039] (1) Put 2 g of dicyandiamide into a semi-closed alumina crucible, raise the temperature to 350 oC at a rate of 2.9 oC per minute under nitrogen atmosphere, keep at this temperature for 2 h, and then The heating rate was 3.3 oC, the temperature was raised to 540 oC, kept at this temperature for 2 h, and then dropped to room temperature naturally. The obtained product was washed four times with deionized water and absolute ethanol, and dried at 50 oC for 12 h to obtain graphite carbon nitride as a yellow solid powder.
[0040] (2) Add 150 mg NH 4 Dissolve Cl into 20 mL deionized water to form NH 4 Cl solution. Immediately followed by 50 mg g-C 3 N 4 Dispersed to the above NH 4 Cl solution, stirred for 30 min, and then sonicated for 30 min to form g-C 3 N 4 Dispersions. The above suspension was transferred to a polytetrafluoroethylene-lined reactor, reacted at 160 °C for 12 h, and then cooled to room temperature naturally. The final product was washed 4 times wit...
Embodiment 3
[0053] (1) Put 2 g of dicyandiamide into a semi-closed alumina crucible, raise the temperature to 350 oC at a rate of 2.9 oC per minute under nitrogen atmosphere, keep at this temperature for 2 h, and then The heating rate was 3.3 oC, the temperature was raised to 540 oC, kept at this temperature for 2 h, and then dropped to room temperature naturally. The obtained product was washed four times with deionized water and absolute ethanol, and dried at 50 oC for 12 h to obtain graphite carbon nitride as a yellow solid powder.
[0054] (2) Add 160 mg NH 4 Dissolve Cl into 20 mL deionized water to form NH 4 Cl solution. Immediately followed by 60 mg g-C 3 N 4 Dispersed to the above NH 4 Cl solution, stirred for 30 min, and then sonicated for 30 min to form g-C 3 N 4 Dispersions. The above suspension was transferred to a polytetrafluoroethylene-lined reactor, reacted at 160 °C for 12 h, and then cooled to room temperature naturally. The final product was washed 4 times wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com