Fluorescent probe for detecting intracellular hydrogen sulfide based on nitroreduction and application thereof
A fluorescent probe and hydrogen sulfide technology, applied in the field of fluorescent probes for detecting hydrogen sulfide (H2S), can solve the problems of damaging biological samples, slow response of hydrogen sulfide, and inability to detect H2, so as to improve detection accuracy and reduce interference Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1. The synthesis of the compound shown in specific formula I.
[0084] Such as figure 2 As shown, the structure of the probe compound used in the specific formula I of the embodiment is coded Cy-NO 2 express
[0085] Dissolve 0.696g m-nitrophenol and 0.0524-0.173g sodium hydride in 30mL anhydrous DMF, add 0.5g cyanine dye Cy7-Cl, and react at room temperature for 24h under nitrogen protection. After the reaction, the DMF was removed in vacuo, purified by silica gel chromatography, and the eluents were ethyl acetate and methanol to obtain 0.425 g of dark green product with a yield of 73.2%. 1 H NMR (500MHz, CDCl 3 -D 1 )δ(ppm):1.25-2.09(m,20H),2.63-2.65(q,4H),4.17-4.22(q,4H),6.08-6.17(d,2H),7.04-7.98(m,12H) ,8.01-8.09(d,2H). 13 C NMR (500MHz, CDCl 3 -D 1 )δ (ppm): 171.4, 162.2, 159.7, 141.7, 141.1, 131.7, 129.9, 128.8, 125.3, 122.3, 122.2, 117.4, 110.7, 110.5, 110.2, 100.7, 77.3, 77.0, 76.8, 40, 28.4, 40. 27.8, 24.8, 21.0, 12.4, 12.3. LC-MS (API-ES)...
Embodiment 2
[0086] Synthesis of compound shown in embodiment 2 concrete formula II
[0087] Such as figure 2 As shown, the structure of the probe compound used in the embodiment specific formula I is coded mCy-NO 2 express
[0088] Dissolve 0.696g m-nitroaniline in 30mL anhydrous DMF, add triethylamine, stir at room temperature for 15min, then add 0.50g cyanine dye Cy7-Cl, react at 60-90℃ for 24h under nitrogen protection. After the reaction, the DMF was removed in vacuo, and purified by silica gel column chromatography, and the eluents were ethyl acetate and methanol. 0.274 g of dark blue product was obtained, with a yield of 47.3%. 1 HNMR (400MHz, CDCl 3 -D 1 ,0.1%CD 3 COOD-D 4 )δ(ppm):8.00(s,1H),7.30-7.15(m,8H),6.50(s,1H),5.84(s,1H),5.28(s,1H),4.02(m,2H), 2.96(s,2H),2.88(s,2H),2.79(s,1H),1.87-1.58(m,10H). 13 C NMR (100MHz, CDCl 3 -D 1 )δ (ppm): 168.8, 162.6, 142.4, 140.7, 140.1, 128.5, 127.7, 123.8, 122.2, 122.0, 121.5, 120.0, 109.2, 105.54, 96.3, 59.2, 54.6, 45.5, 39.3, 3...
Embodiment 3
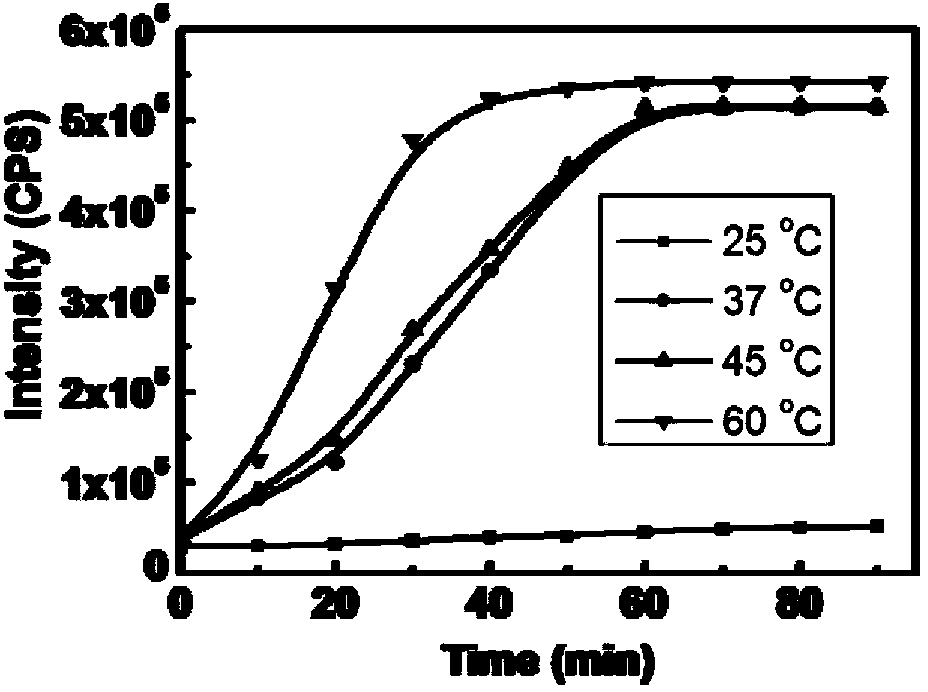
[0091] Compound Cy-NO shown in specific formula I 2 Temperature Effects on the Response to Hydrogen Sulfide
[0092] In order to simulate physiological conditions as much as possible, the following detection effect experiments were carried out under the condition of pH=7.4 (HEPES buffer solution, the concentration was 40mM), and the probe concentration was 10μM.
[0093] pH was controlled with HEPES buffer solution. Add 10 μM Cy-NO, the compound shown in specific formula Ⅰ, to the 10ml colorimetric tube 2 , then 40 mM HEPES was added, followed by 350 μM Na 2 S, dilute to 10ml with ultrapure water, shake the solution evenly, and equilibrate at 25, 37, 45, and 60°C for 50 minutes, then add the above working solution into a fluorescent dish to measure the fluorescence spectrum. . Fluorescence intensity changes with temperature as Figure 4 shown. Figure 4 show that Cy-NO 2 and H 2 The reaction rate of S is affected by temperature. For the purpose of in vivo application ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com