Industrial production method of high-purity sulfuric acid terbutaline
A technology of terbutaline sulfate and a synthesis method, applied in the field of medicine, can solve the problems of complicated operation, large environmental pollution of by-products, harm to the life of employees, etc., and achieves the effects of mild reaction conditions, high product purity and little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
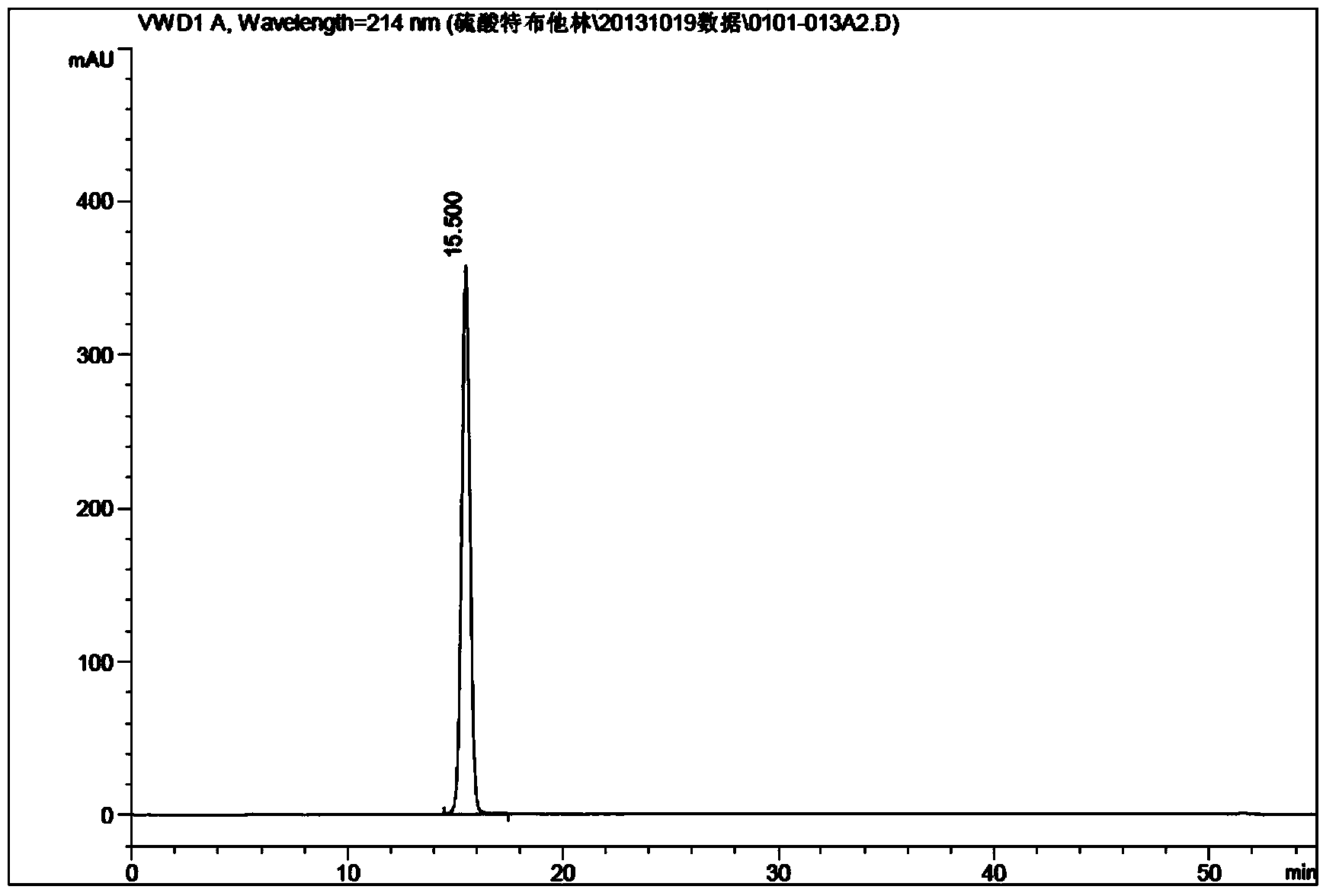
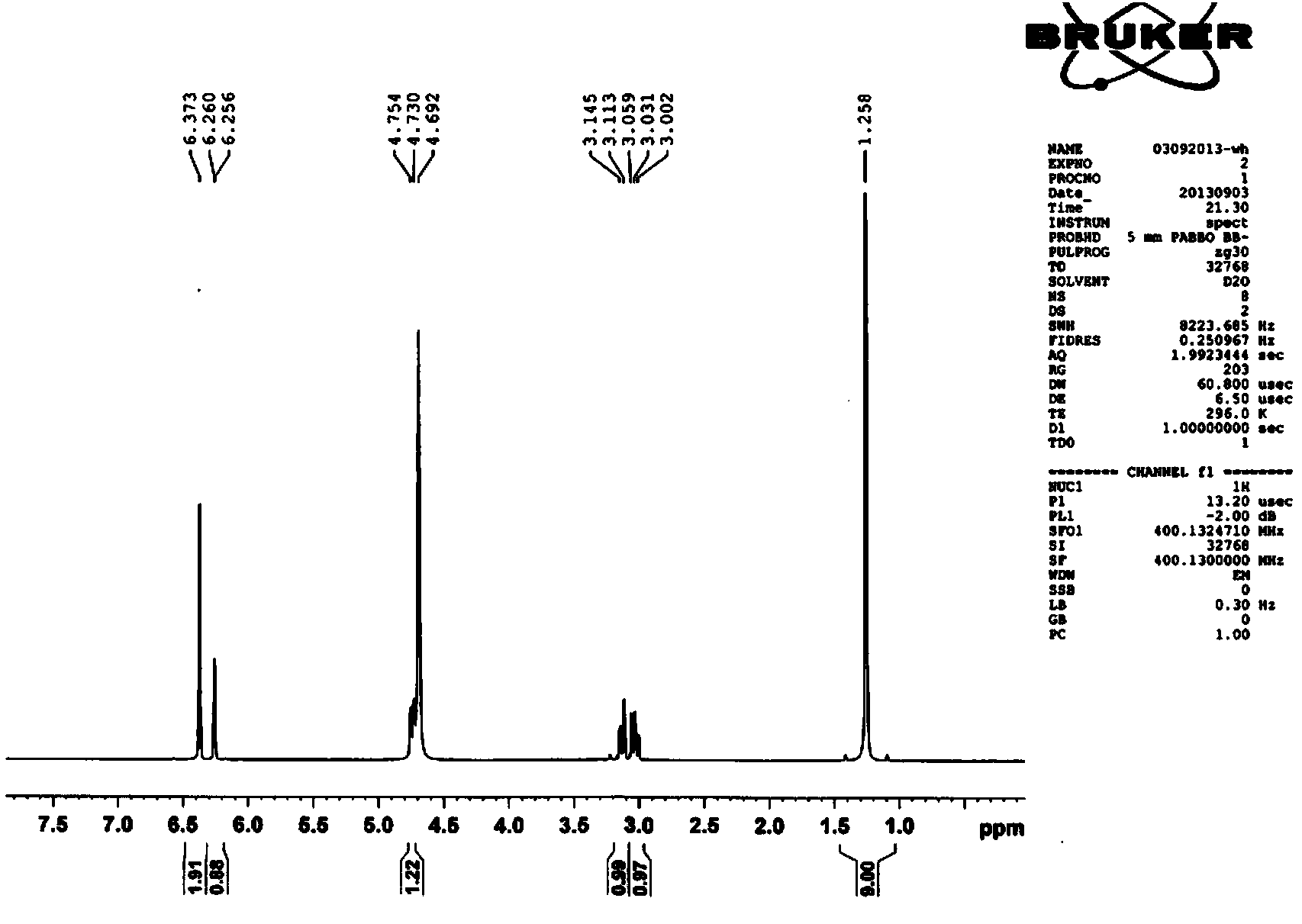
Examples
Embodiment 1
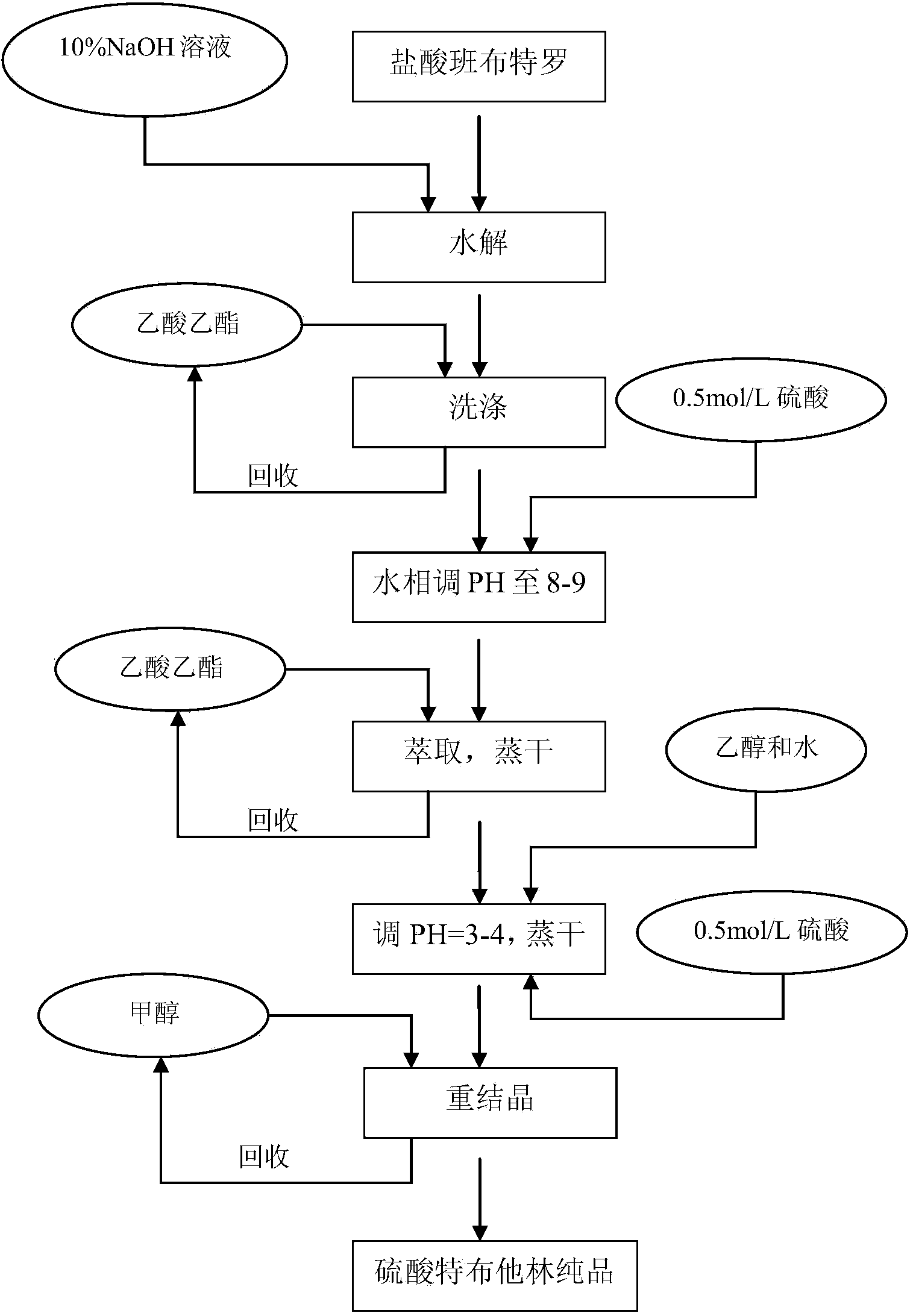
[0022] Embodiment 1 (see attached figure 1 )
[0023] Synthesis process and refining method of terbutaline sulfate:
[0024] 1) Preparation of crude terbutaline sulfate:
[0025] Add 20kg of bambuterol hydrochloride into 200L of 10% sodium hydroxide aqueous solution, stir for 10 minutes, pass nitrogen protection, and reflux at 105°C for 8 hours (TLC detects that the reaction is complete). Check the gas outlet with PH test paper until no alkaline gas is released.
[0026] Cool the reaction liquid, distill it under reduced pressure to 100L, add 100L ethyl acetate to the water phase and wash twice, discard the ethyl acetate phase, adjust the pH of the water phase to 8-9 with 0.5mol / L sulfuric acid, add 200L ethyl acetate to extract three times , combine the organic phases, evaporate to dryness under reduced pressure, add 50L water for injection and 50L ethanol to dissolve the residue, add 0.5mol / L sulfuric acid to adjust the pH=3-4, and evaporate to dryness under reduced press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com