Dispersion preparation containing dabigatran etexilate
A technology of dabigatran etexilate and dispersant, applied in the field of medicine, can solve the problems of complex operation process, complex process operation, uneven drug application and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
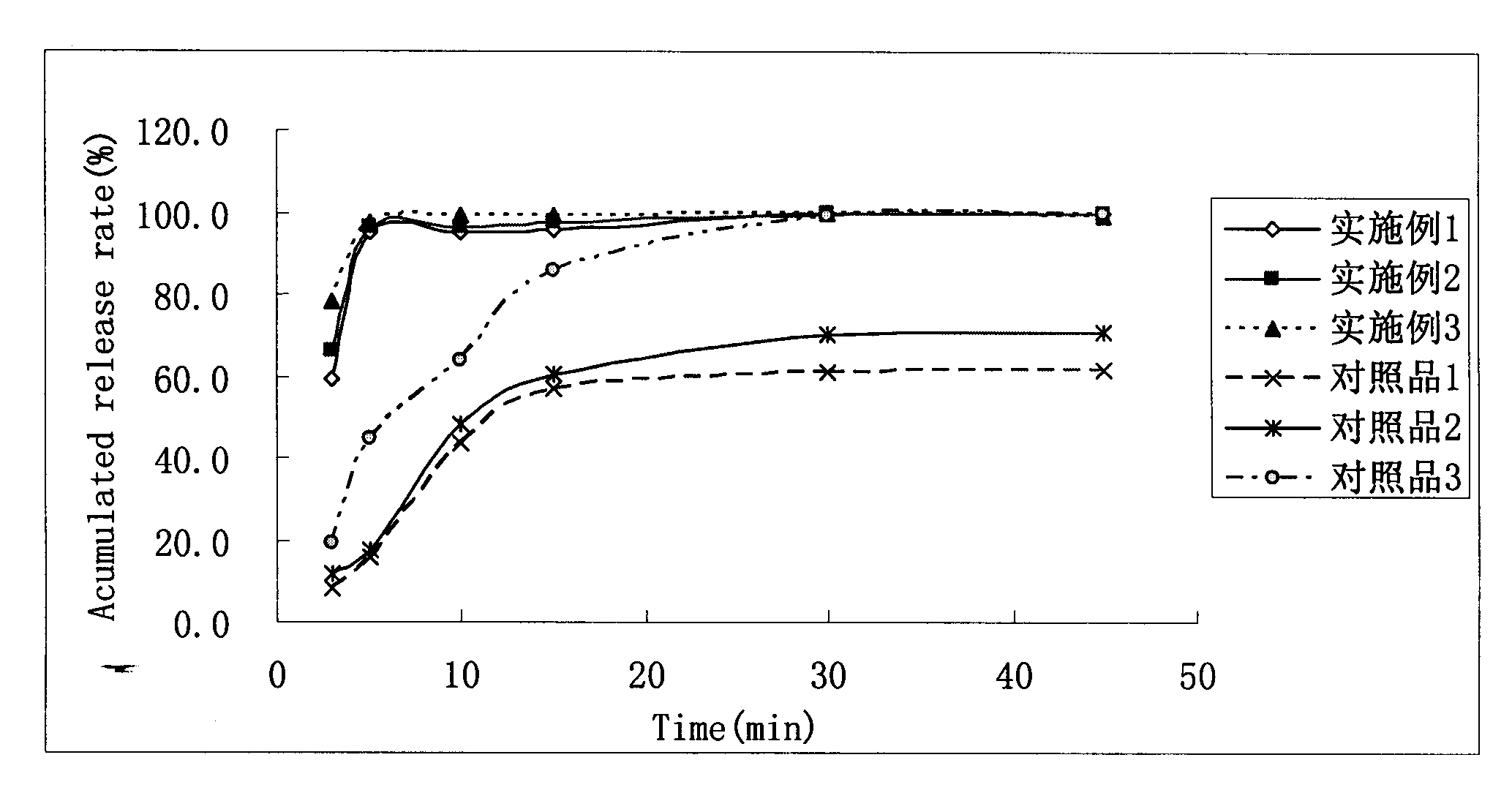
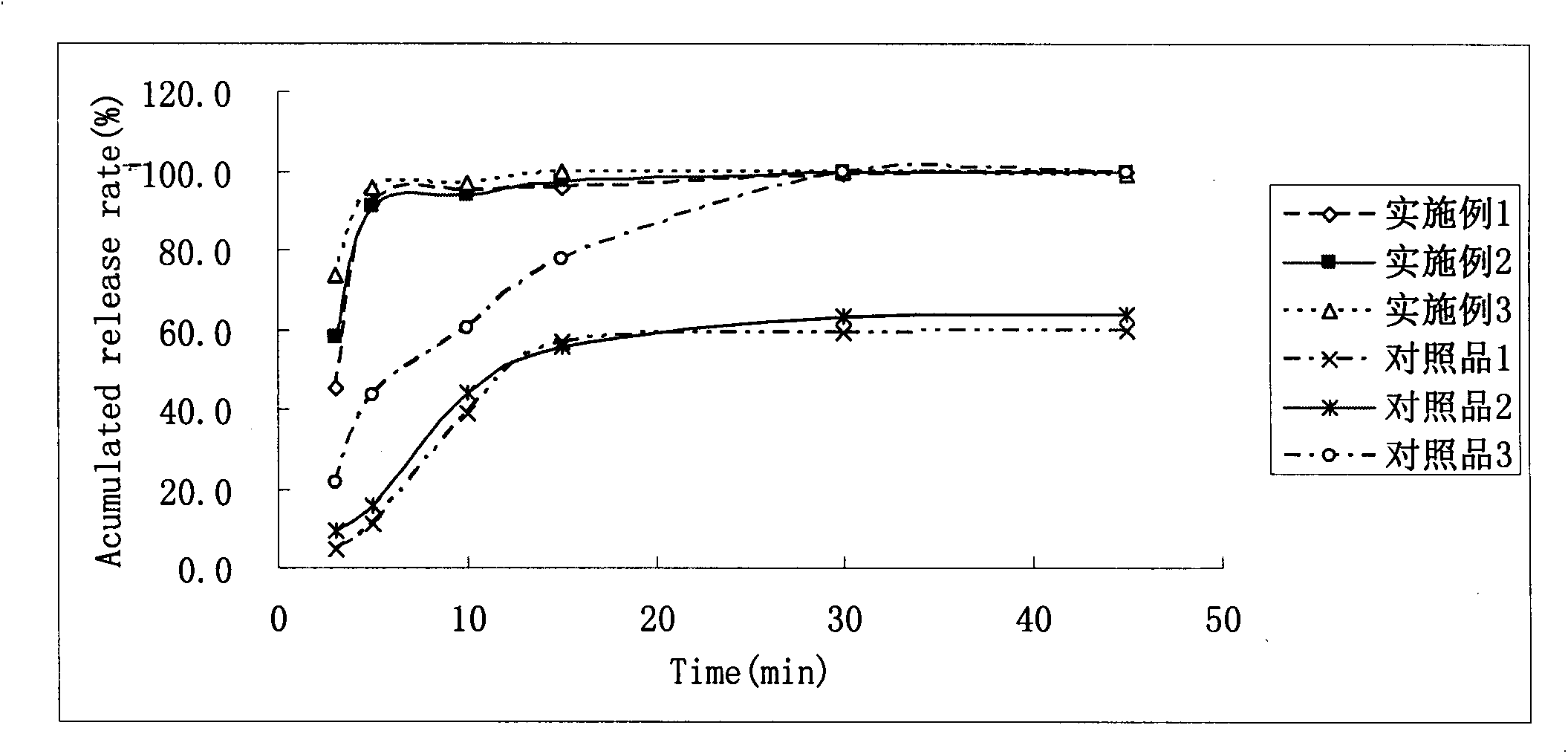
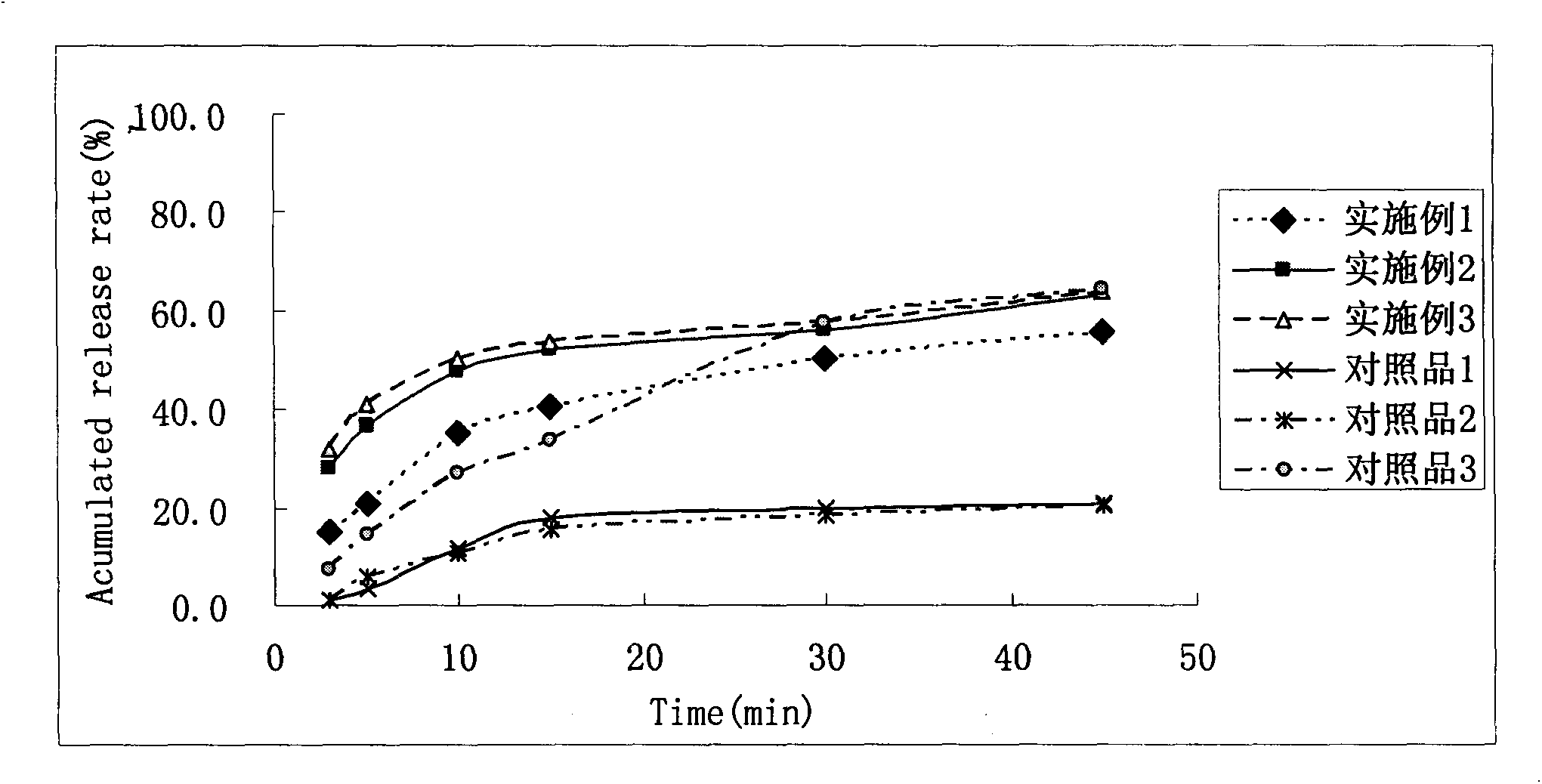
Image
Examples
Embodiment 1
[0036] Embodiment 1: Dabigatran etexilate dispersible tablet
[0037] Specifications: 1000 pieces
[0038]
[0039]
[0040] Coating Solution Prescription
[0041] Component Dosage
[0042] Opadry Coating Solution (03K19229) 100 parts
[0043] Purified water 1000ml
[0044] Preparation:
[0045] 1. Accurately weigh all the raw materials and auxiliary materials according to the prescription amount, and pass through a 100-mesh sieve. The disintegrant is divided into internally added and externally added parts, and the ratio of internally added and externally added parts is 4:1 (weight ratio); The extra parts are weighed and marked separately;
[0046] 2. Put the dabigatran etexilate mesylate raw material and all the internally added auxiliary materials in the mixer evenly; add the binder, continue mixing to make a suitable soft material, and granulate with a 30-mesh sieve;
[0047] 3. After the granules are dried at 40°C to 45°C, the water content is determi...
Embodiment 2
[0050] Embodiment 2 Dabigatran etexilate dispersible tablet
[0051] 1. Accurately weigh all the raw materials and auxiliary materials according to the prescription quantity, and pass through a 100-mesh sieve. The disintegrant is divided into internally added and externally added parts. The ratio of internally added and externally added parts is 3:2, and the weight ratio; internally added and externally added Parts are weighed and marked separately;
[0052] 2. Put the dabigatran etexilate mesylate raw material and all the internally added auxiliary materials in the mixer evenly; add the binder, continue mixing to make a suitable soft material, and granulate with a 30-mesh sieve;
[0053] 3. After the granules are dried at 40°C to 45°C, the water content is determined to be qualified, and the granules are sieved with a 30-mesh sieve.
[0054] 4. Add all the excipients according to the prescription amount, mix well, measure the particle content, calculate the tablet weight, ...
Embodiment 3
[0056] Embodiment 3 Dabigatran etexilate mesylate dispersible granule capsule
[0057] Specifications: 1000 pieces
[0058] Prescription:
[0059]
[0060] 1. Accurately weigh all raw materials and auxiliary materials according to the prescription amount, and pass through a 100-mesh sieve.
[0061] 2. Put the dabigatran etexilate mesylate raw material and all the internally added auxiliary materials in the mixer evenly; add the binder and continue mixing to make a suitable
[0062] 3. Soft material, granulated with 60 mesh sieve;
[0063] 4. After the granules are dried at 40°C to 45°C, the water content is determined to be qualified, and the granules are sieved with a 60-mesh sieve.
[0064] 5. Calculate the drug content of the obtained granules, weigh them accurately, and fill them in hollow capsules to obtain. The active substance content of each dabigatran etexilate sulfonate dispersible granule capsule is 150mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com