Analysis method for four anthraquinones in blood plasma and application of four anthraquinones in pharmacokinetics
A blood plasma and method quantitative technology, applied in the direction of analysis of materials, scientific instruments, material separation, etc., can solve the problem of simultaneous detection and achieve high sensitivity and accurate detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] LC-MS / MS conditions
[0117] Column: Hypersil Gold-C 18 (150×2.1mm, 5μm, Thermo)
[0118] Pre-column: Security Guard-C 18 (4.0mm×3.0mm i.d,5μm,Phenomenex)
[0119] Mobile phase: acetonitrile-0.05% ammonia solution (70:30)
[0120] Column temperature: 25℃
[0121]Flow rate: 250μL·min -1
[0122] Ion source: Electrospray ionization source (ESI)
[0123] Ionization mode: negative ion mode
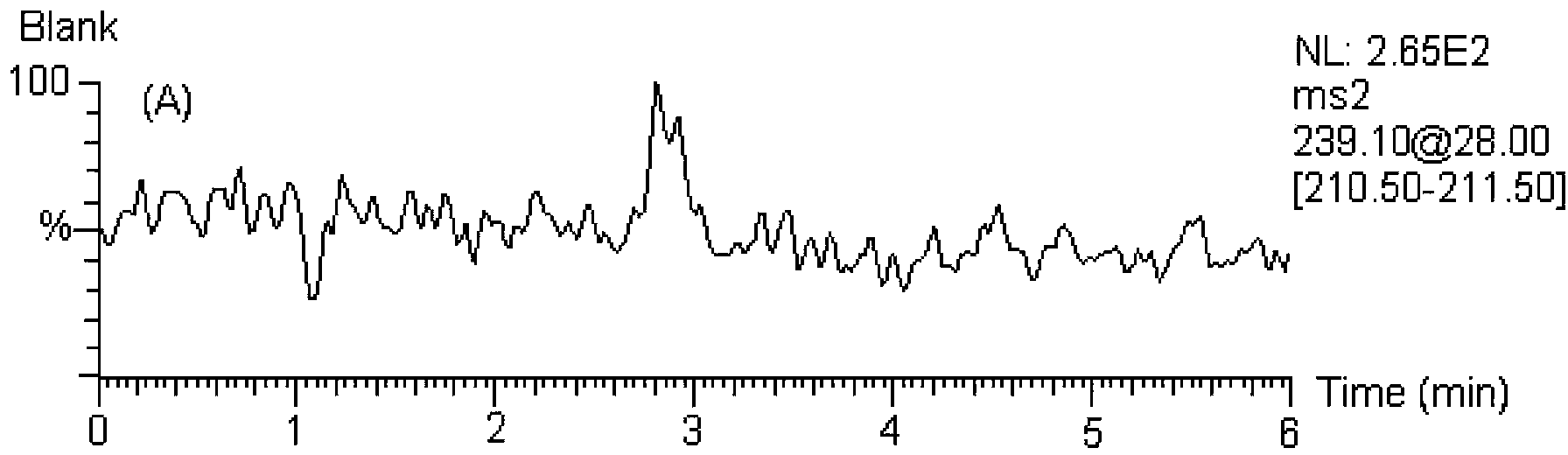
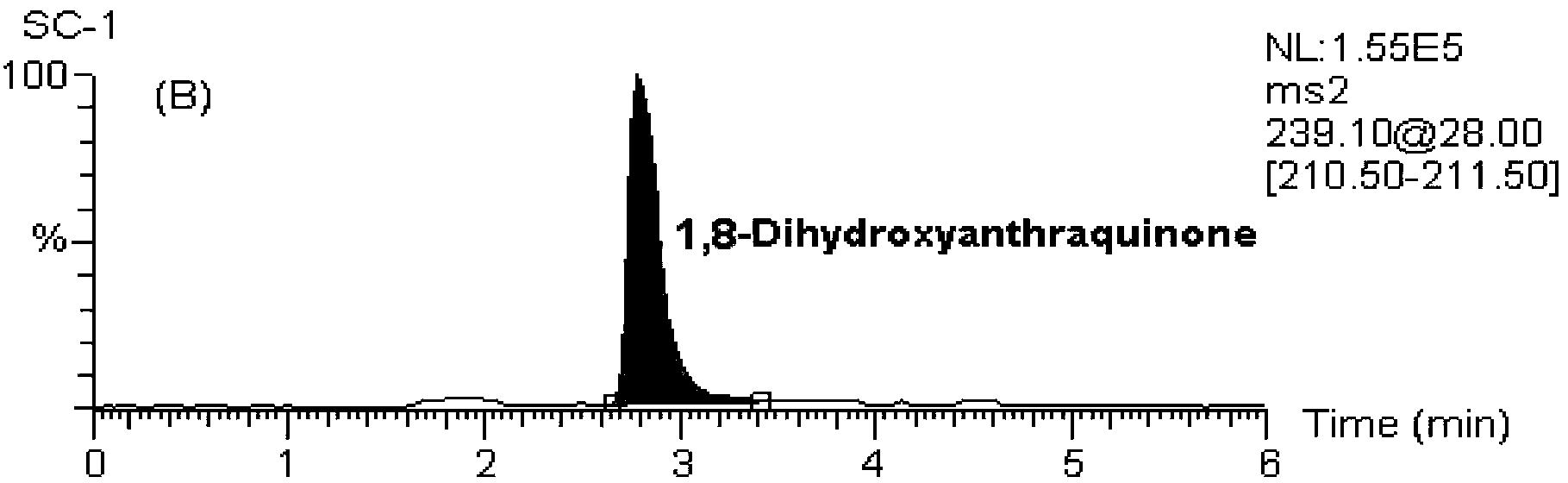
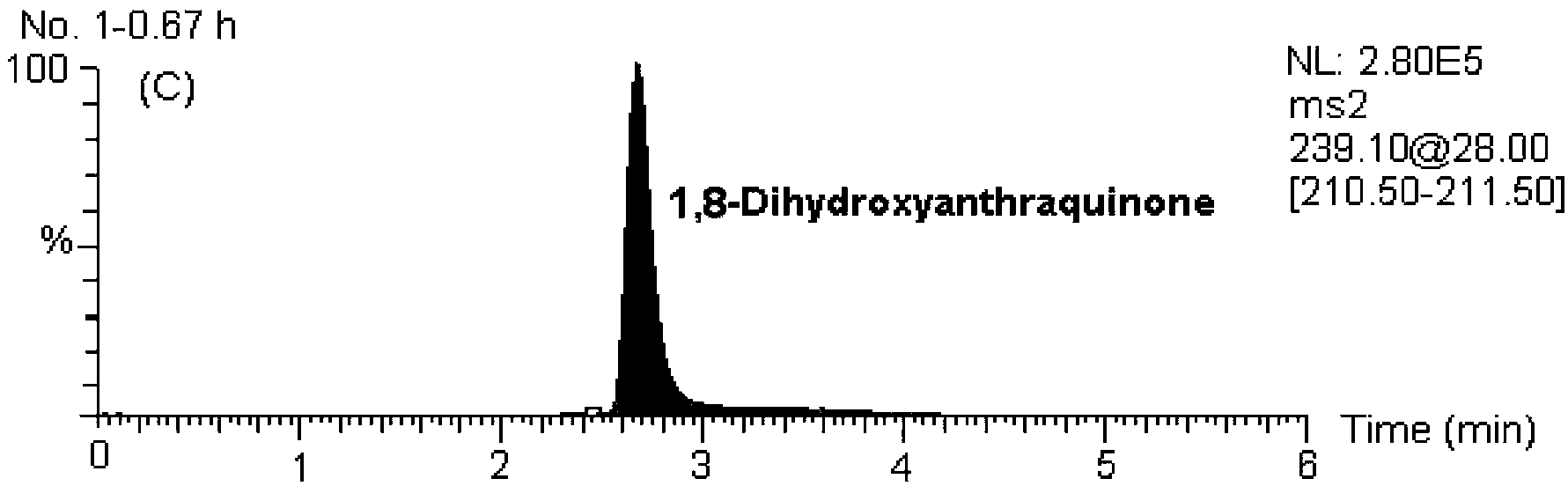
[0124] MS / MS: Selective Reaction Monitoring (SRM). The ion reactions used for quantitative analysis were m / z239→211 (internal standard, 1,8-dihydroxyanthraquinone), m / z253→225 (chrysophanol), m / z269→225 (emodin), m / z z269→240 (Aloe-emodin), m / z283→240 (Emodin methyl ether).
[0125] Mass spectrometry parameters: spray voltage: 3500V; ion source voltage: 10eV; capillary temperature: 350°C; sheath gas: N 2 (35psi); auxiliary gas: N 2 (10psi); collision gas pressure: Ar1.0mTorr (1Torr=133.3Pa); collision-induced fragmentation (CID) voltages of 1,8-dihydroxyanthraquinone, chryso...
Embodiment 2
[0134] LC-MS / MS conditions
[0135] Column: Hypersil Gold-C 18 (150×2.1mm, 5μm, Thermo)
[0136] Pre-column: Security Guard-C 18 (4.0mm×3.0mm i.d,5μm,Phenomenex)
[0137] Mobile phase: acetonitrile-0.05% ammonia solution (70:30)
[0138] Column temperature: 25℃
[0139] Flow rate: 250μL·min -1
[0140] Ion source: Electrospray ionization source (ESI)
[0141] Ionization mode: negative ion mode
[0142] MS / MS: Selective Reaction Monitoring (SRM). The ion reactions used for quantitative analysis were m / z239→211 (internal standard, 1,8-dihydroxyanthraquinone), m / z253→225 (chrysophanol), m / z269→225 (emodin), m / z z269→240 (Aloe-emodin), m / z283→240 (Emodin methyl ether).
[0143] Mass spectrometry parameters: spray voltage: 3000V; ion source voltage: 10eV; capillary temperature: 350°C; sheath gas: N 2 (35psi); auxiliary gas: N 2 (20psi); collision gas pressure: Ar1.0mTorr (1Torr=133.3Pa); collision-induced fragmentation (CID) voltages of 1,8-dihydroxyanthraquinone, chrys...
Embodiment 3
[0150] LC-MS / MS conditions
[0151] Column: Hypersil Gold-C 18 (100×2.1mm, 5μm, Thermo)
[0152] Pre-column: Security Guard-C 18 (4.0mm×3.0mm i.d,5μm,Phenomenex)
[0153] Mobile phase: acetonitrile-0.05% ammonia solution (70:30)
[0154] Column temperature: 25℃
[0155] Flow rate: 300μL·min -1
[0156] Ion source: Electrospray ionization source (ESI)
[0157] Ionization mode: negative ion mode
[0158] MS / MS: Selective Reaction Monitoring (SRM). The ion reactions used for quantitative analysis were m / z239→211 (internal standard, 1,8-dihydroxyanthraquinone), m / z253→225 (chrysophanol), m / z269→225 (emodin), m / z z269→240 (Aloe-emodin), m / z283→240 (Emodin methyl ether).
[0159] Mass spectrometry parameters: spray voltage: 2500V; ion source voltage: 10eV; capillary temperature: 350°C; sheath gas: N 2 (45psi); auxiliary gas: N 2 (15psi); collision gas pressure: Ar1.0mTorr (1Torr=133.3Pa); collision-induced fragmentation (CID) voltages of 1,8-dihydroxyanthraquinone, chrys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com