Tumor nucleic acid vaccine based on tissue factor as well as preparation method and application of vaccine
A tumor nucleic acid vaccine and tissue factor technology, applied in the field of preparation and nucleic acid vaccines, can solve problems such as difficult process, complex protein structure, allergic reaction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
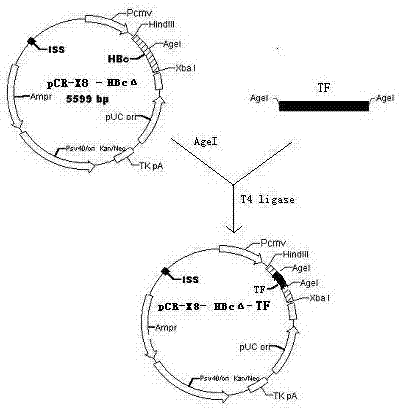
[0020] Example 1: Construction of eukaryotic expression vector pCR-X8-HBcΔ-TF
[0021] 1.1 Construction of eukaryotic expression vector pCR-X8-HBcΔ-TF
[0022] 1.1.1 Cloning of TF target gene
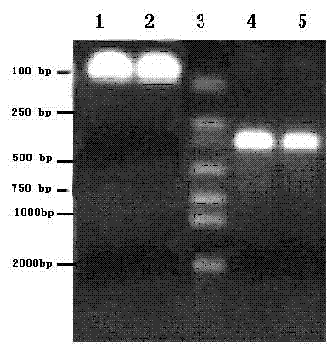
[0023] Gene cloning of TF target fragment
[0024] Design upstream and downstream primers as templates for each other, and obtain target fragments by PCR
[0025] TF
[0026] Upstream primer TF-up: 5'- T GGT ACC GGT GAC TGG AAA TCC AAA TGC TTC TAC ACT ACT GAC A-3' (SEQ ID NO: 2)
[0027] Downstream primer TF-down: 5'- AAA AAGC TTA ACC GGT TTC GTC AGT CAA GTC GTA TTC AGT GTC AGT AGT GTA GAA GCA T-3' (SEQ ID NO: 3)
[0028] Both upstream and downstream primers contain restriction endonuclease AgeI sites (indicated by the shaded part)
[0029] PCR reaction system:
[0030] 10×PFU PCR buffer 10 μl
[0031] TF cDNA 2 μl
[0032] 10×PFU PCR buffer 10 μl
[0033] Upstream primer 8 μl
[0034] Downstream primer 8 μl
[0035] dNTP 8 μl
[0036] PFU DNA Polymerase 1 μl
[0...
Embodiment 2
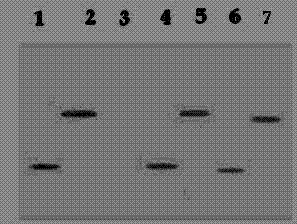
[0067] Example 2: ELISA detection and Western blot detection of TF antibody
[0068] 2.1 Materials
[0069] 2.1.1 Experimental animals
[0070] C57 / BL6 pure line mice, female, 6-8 weeks old, with an average body weight of 16.8 g before immunization, were purchased from the Experimental Animal Center of Yangzhou University.
[0071] experimental drug
[0072] Recombinant nucleic acid vaccine HBcΔ-TF.
[0073] Materials and Reagents
[0074] Purified recombinant proteins VEGF-TFh, VEGF-TFM, VEGF-PVH, VEGF-PVM. The specific test operation is the same as before.
[0075] Materials and Reagents
[0076] Purified recombinant proteins VEGF-TF, VEGF-PV. The specific test operation is the same as before.
[0077] method
[0078] 2.2.1 Immunization program
[0079] C57BL / 6J female mice were selected and divided into random groups, 10 mice in each group, including normal saline control group, plasmid vector control group and pCR-X8-HBcΔ-TF group. Nucleic ac...
Embodiment 3
[0089] Example 3: Research on anti-tumor effect of recombinant TF subunit epitope nucleic acid vaccine
[0090] 3.1 Tumor cell culture and transplantation tumor model preparation
[0091] 3.1.1 Materials
[0092] B16-F10 was purchased from the Institute of Cells, Chinese Academy of Sciences, RPMI1640 and DMEM medium were purchased from JIBCOL, and C57 / BL6 mice were purchased from the Comparative Medicine Center of Yangzhou University.
[0093] Cell Culture and Transplantation
[0094] B16-F10 is a highly metastatic melanoma strain with a C57 / BL6 background, and it is also an adherent growth cell. The culture medium is DMEM medium containing 10% calf serum, and it is inoculated in a culture flask. 5%CO 2When the cells grow into a continuous monolayer under certain conditions, carefully pour off the medium, gently wash with D-HANKS solution, pour off the D-HANKS solution, and add 0.125% trypsin to digest. Pour off the trypsin, immediately add 10% calf serum 1640 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com