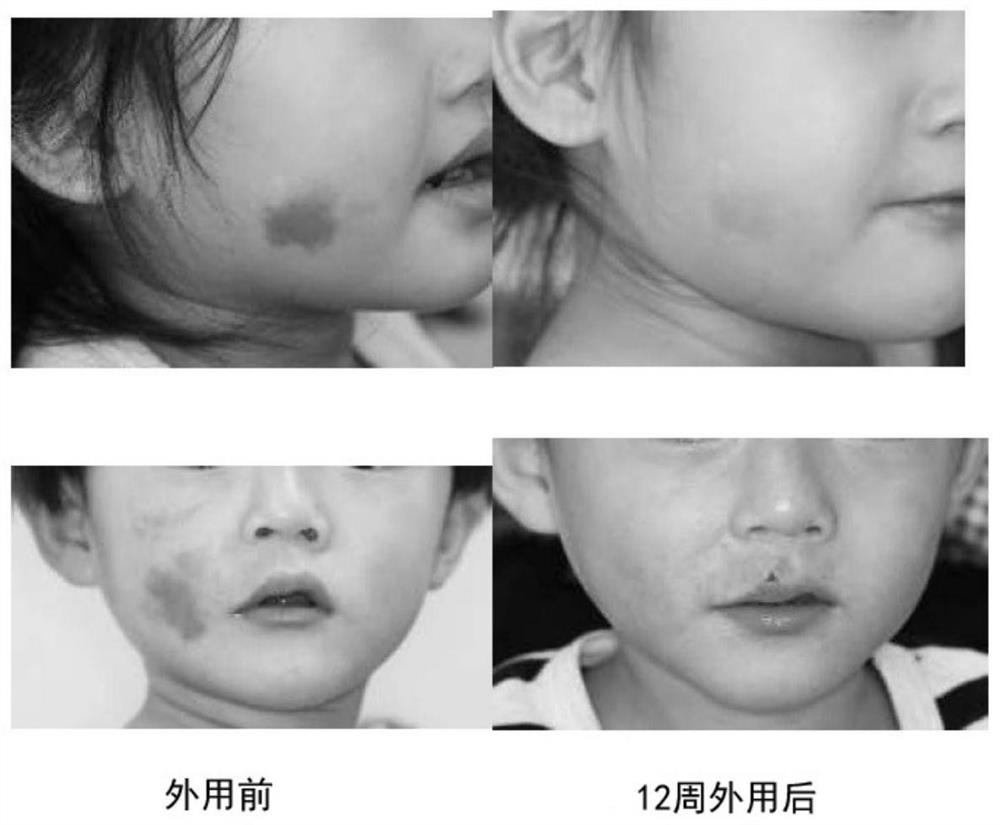
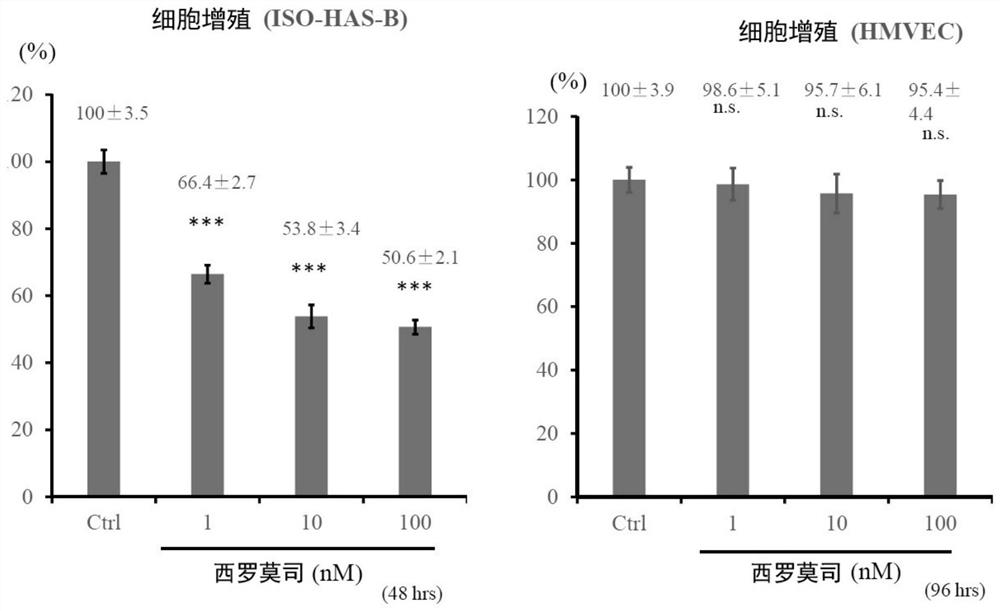
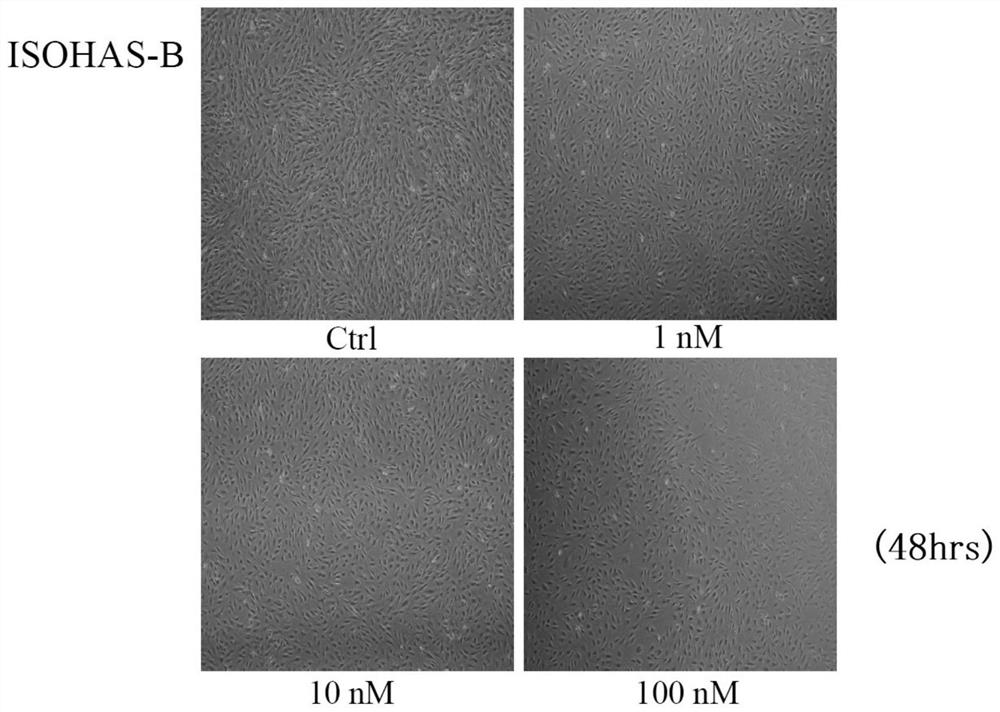
External preparation for vascular abnormality treatment
An external agent and vascular technology, applied in the direction of cardiovascular system diseases, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as vascular malformations with unknown causes, achieve simple administration, safe treatment, and eliminate increased blood vessels Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] Examples of the preparation method of the external preparation include the following. (i) A gel can be prepared by gelling a solution containing sirolimus or a sirolimus derivative. (ii) An ointment can be prepared by mixing an ointment base with sirolimus or a sirolimus derivative. (iii) Patches, cataplasms, liniments, lotions and creams can be prepared according to known methods.
[0070] Hereinafter, gels, ointments, and creams will be specifically described.
[0071] (1) Gel
[0072] The external preparation for treating vascular abnormalities of the present invention may be a gel containing sirolimus or a sirolimus derivative.
[0073] When gelling a solution containing sirolimus or a sirolimus derivative, the solution may be gelled using a gelling derivatizing agent. Examples of the gelling derivatizing agent include carboxyvinyl polymers, carboxymethylcellulose, aluminum hydroxide, bentonite and the like.
[0074] The specific constitution of the carboxyviny...
manufacture example
[0135] [Manufacturing example: Preparation of sirolimus-containing gel]
[0136] Gels containing various concentrations of sirolimus were prepared. As a specific method, after adding and dissolving sirolimus in ethanol, water for injection was added and mixed to prepare a mixed solution. Carbomer (registered trademark) 934P NF was added and mixed to the mixed solution to prepare a homogeneous suspension. Tris was added and mixed as a neutralizing agent to the suspension to prepare a gel.
[0137] In addition, the components in 1 g of the gel other than sirolimus were 16 mg of Carbomer (registered trademark) 934P NF, 490 mg of water, 480 to 490 mg of ethanol, and 6 mg of tris.
[0138] More specifically, gels were prepared with the compositions of Production Examples 1 to 5 below.
manufacture example 1
[0139] Production Example 1: Gel containing 0.4% by weight of sirolimus (100 g in total)
[0140] Sirolimus 0.4g
[0141] Ethanol 48.4g
[0142] Water for injection 49g
[0143] Carbomer (registered trademark) 934P NF 1.6g
[0144] Trishydroxymethylaminomethane 0.6g
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com