Orange optical iridium coordination compounds and application thereof in organic electro-generated white or orange optical device
A technology of orange iridium and complexes, applied in the field of organic electroluminescence, can solve problems such as high LUMO energy level, unsatisfactory, and reduced bandgap width, so as to increase electroluminescence efficiency, increase charge transport balance performance, The effect of reducing vibration frequency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: Complex (CF 3 P-CF 3 BT) 2 Synthesis of Ir(acac)
[0049] The specific synthesis steps are as follows:
[0050]
[0051] 1: Ligand CF 3 P-CF 3 Synthesis of BT
[0052] Synthesis of 1: Dissolve 4-aminobenzotrifluoride (10.08g, 62.60mmol), 4-trifluoromethylbenzoyl chloride (10.00g, 62.60mol), and triethylamine (9.6mL) in 100ml of chloroform. At 0°C, react under the protection of argon for 30 min, then transfer to room temperature to continue the reaction for 6 h, remove the solvent, and recrystallize with ethanol to obtain 15.9 g (80%) of white flocculent solid. 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 7.98 (d, J = 7.7 Hz, 2H), 7.90 (s, 0H), 7.77 (d, J = 7.9 Hz, 4H), 7.64 (d, J = 8.2 Hz, 2H). 13 C NMR (101 MHz, CDCl 3 ): δ (ppm) 164.37, 144.13, 140.58, 137.69, 133.79, 127.58, 126.52, 126.48, 126.06, 126.02, 119.88.
[0053] Synthesis of 2: Dissolve 1 (13.34g, 40mol) and Lawson's reagent (22.08g, 60mmol) in 100mL of toluene, vacuumize / argon flow for ...
Embodiment 2
[0057] Example 2: In this example, the suitable R in the compound of formula 1 1 -R 4 The groups include the substituent configurations in Table 1.
[0058] Table 1
[0059]
[0060]
[0061]
Embodiment 3
[0062] Embodiment 3: the preparation of orange light organic light-emitting diode
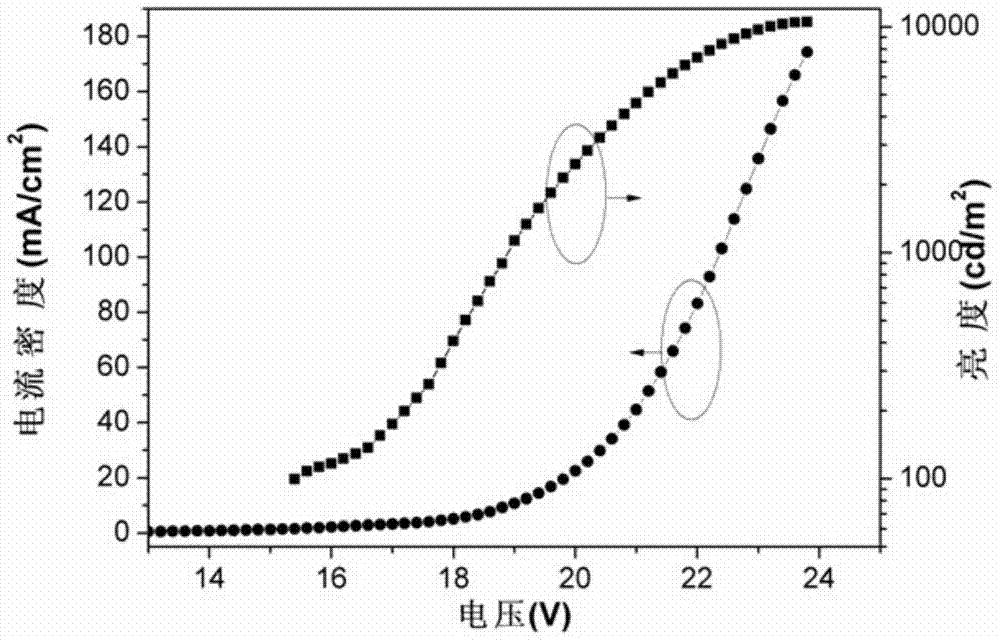
[0063]For the given orange light embodiment, the structure of the device is: ITO / PEDOT:PSS (40nm) / PVK:OXD-7: (CF 3 P-CF 3 BT) 2 Ir(acac) (40nm) / Ca(10nm) / Al(100nm), the preparation process of the device is as follows:
[0064] 1. Spin-coat the conductive polymer PEDOT:PSS on the anode ITO at 3000r / min, and anneal at 120°C for 1h to form a 40nm-thick hole-injecting double-layer electrode;
[0065] 2. Add PVK:OXD-7:(CF 3 P-CF 3 BT) 2 Ir(acac) was made into a solution with a concentration of 15 mg / ml at 100:40:10, spin-coated on PEDOT:PSS at a speed of 2000 rpm, and annealed at 120°C for 10 minutes in nitrogen;
[0066] 3. Evaporate Ca(10nm) / Al(100nm) on the light-emitting layer as the cathode.
[0067] The properties of the obtained orange EL device are as follows: turn-on voltage: 8V, maximum brightness: 10527cd / m 2 , the highest efficiency: 11.03 cd / A (19.8V), color coordinates (0.48,0.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com