Oil-in-water-in-oil type inactivated vaccine for avian influenza virus and preparation method thereof
A bird flu virus, water-in-oil-in-water technology, applied in antiviral agents, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve problems such as high production costs, biosafety, and slow antibody production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
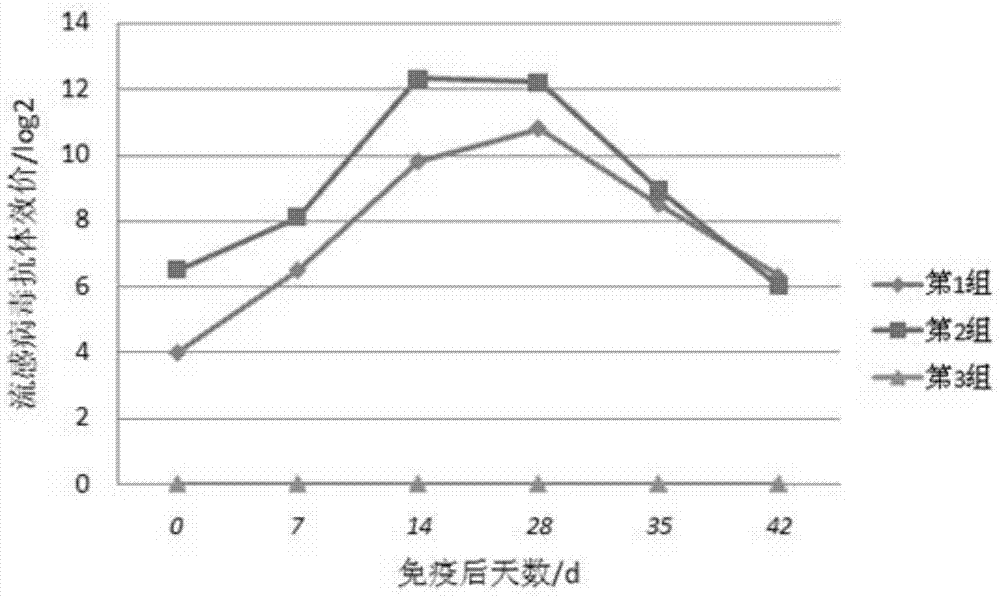
Image
Examples
Embodiment 1
[0014] 1.1 Virus cultivation and harvest
[0015] Take the well-grown MDCK cells in the cell culture flask, digest them with EDTA-trypsin cell dispersion solution to prepare cell suspension, count the cells as 8×10 5 Cell density per mL was inoculated in DMEM medium containing 8% fetal bovine serum, and cultured overnight in shake flasks at 37°C; when the cells covered the monolayer of the medium, the inoculation amount of the seed virus was 0.1 MOI and the virus content was ≥ 10 6.5 TCID 50 / mL HP strain H9 subtype avian influenza virus, control the temperature at 37°C and continue to shake the flask for 48-96 hours, harvest the cell culture, freeze and thaw repeatedly 3 times, and obtain the cell venom containing the supernatant;
[0016] 1.2 Inactivation of virus
[0017] Put the cell venom containing the supernatant obtained above in a sterilized container, add formaldehyde solution according to the volume of the virus liquid and adjust the final concentration of formal...
Embodiment 2
[0050] 1.1 Virus cultivation and harvest
[0051] Take the well-grown MDCK cells in the cell culture flask, digest them with EDTA-trypsin cell dispersion solution to prepare cell suspension, count the cells as 3×10 6 Cell density per mL was inoculated in DMEM medium containing 8% fetal bovine serum, and cultured overnight in shake flasks at 37°C; when the cells covered the monolayer of the medium, the inoculation amount of the seed virus was 0.3 MOI and the virus content was ≥ 10 6.5 TCID 50 / mL HP strain H9 subtype avian influenza virus, control the temperature and continue to shake the flask for 96 hours at 37 ° C, harvest the cell culture, freeze and thaw repeatedly 3 times, and obtain the cell venom containing the supernatant.
[0052] 1.2 Inactivation of virus
[0053] Put the cell venom containing the supernatant obtained above in a sterilized container, add formaldehyde solution according to the volume of the virus liquid and adjust the final concentration of formald...
Embodiment 3
[0057] 1.1 Virus cultivation and harvest
[0058] Take the well-grown MDCK cells in the cell culture flask, digest them with EDTA-trypsin cell dispersion solution to prepare a cell suspension, and count the cells according to 10 6 Cell density per mL was inoculated in DMEM medium containing 8% fetal bovine serum, and cultured overnight in shake flasks at 37°C; when the cells covered the monolayer of the medium, the inoculation amount of the seed virus was 0.05MOI and the virus content was ≥ 10 6.5 TCID 50 / mL HP strain H9 subtype avian influenza virus, control the temperature and continue to shake the flask for 80 hours at 37 ° C, harvest the cell culture, freeze and thaw repeatedly 3 times, and obtain the cell venom containing the supernatant.
[0059] 1.2 Inactivation of virus
[0060] Put the cell venom containing the supernatant obtained above in a sterilized container, add formaldehyde solution according to the volume of the virus liquid and adjust the final concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com