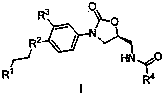
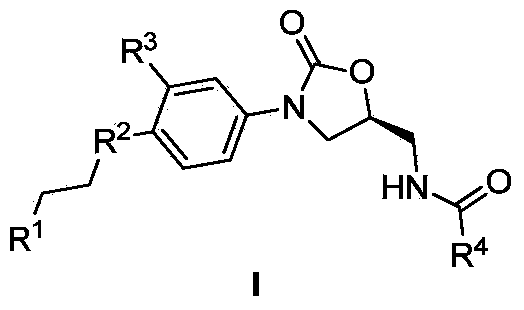
Benzopyrone-phenyl-oxazolidone compounds as well as preparation methods and applications thereof
A technology of benzopyrone and oxazolidinone, which is applied in the field of preparation of antibacterial drugs and can solve the problems of no dual-target antibacterial compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: (R)-N-(4-(4-(3'-hydroxyisoflavone-7-yloxyethyl)piperazin-1-yl)-3-fluorophenyl)-5-acetamide Preparation of methyl-2-oxazolidinone (1)
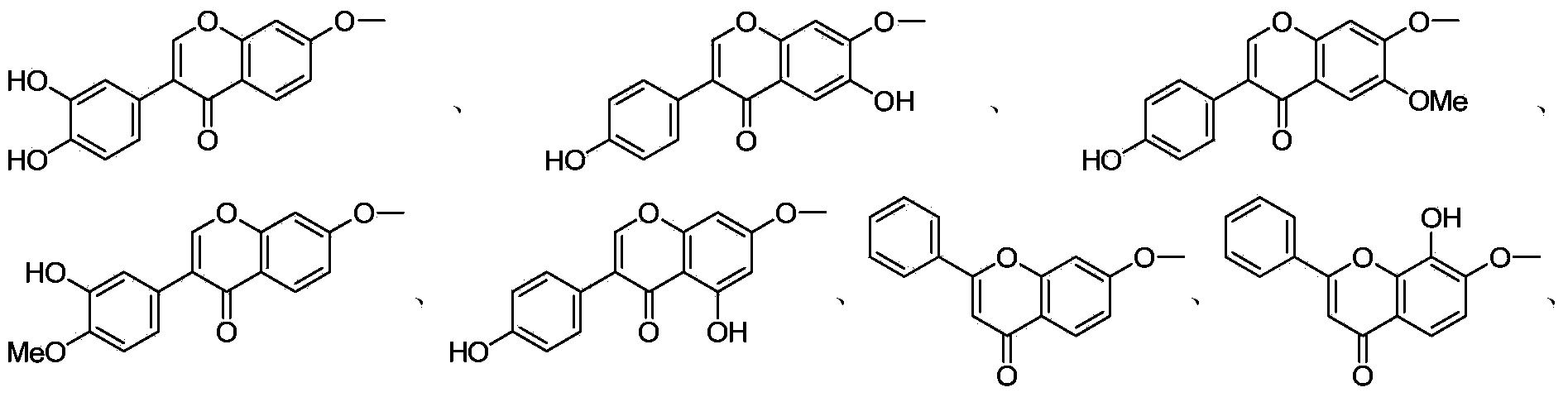
[0022] Step 1: Dissolve 2.54 g (10 mmol) 3′,7-dihydroxyisoflavone in 30 mL DMSO, add 25 mL 1,2-dibromoethane and 5.52 g (40 mmol) K at room temperature 2 CO 3 Raise the temperature to 50°C and react for 12 hours. After the reaction is completed, add water, precipitate out and filter with suction, and use silica gel column chromatography. The eluent is petroleum ether-AcOEt. The volume ratio of petroleum ether and AcOEt is 1:3, and white Solid 3′-hydroxy-7-bromoethoxyisoflavone, yield 79.3%, melting point: 177-179°C;
[0023] Step 2: Add 2.24g (10mmol) 3-fluoro-4-(piperazin-1-yl)benzoic acid and 1.36g (12mmol) methoxyformyl chloride to 7mL (50mmol) triethylamine, and react at room temperature After 1.5h, add 0.78g (12mmol) sodium azide, continue the reaction for 1h, add 1.18g (12mmol) (S)-2-azidomethyloxirane, 0.7g (8mmol) l...
Embodiment 2
[0027] According to the method similar to Example 1, the multi-target aryl-linked benzopyrone-oxazolidinone compounds 1-96 listed in Table 1 were synthesized.
[0028] Each R group of the benzopyrone-oxazolidinone type compound connected by the aryl group in the general formula I of table 1
[0029]
[0030]
[0031]
[0032]
[0033]
[0034]
[0035]
[0036]
[0037]
[0038]
[0039]
[0040] Note: the initial raw materials were purchased from aldrich company
[0041] Example 2: Efflux rate of cellular compounds
[0042] Staphylococcus aureus was inoculated in the liquid medium, cultivated until the OD value (600nm) reached 0.6, the bacterial solution was centrifuged at 6000×g for 5min, and washed with 20mM hydroxyethylpiperazineethanesulfonic acid buffer solution (HEPES, pH7. 0) Wash 3 times, resuspend the cells in the above HEPES buffer solution, control the cell concentration at about 40mg / mL, add the test compound, the compound concentra...
Embodiment 3
[0045] Example 3: Activity of ribosomal protein synthesis
[0046] Take the Escherichia coli liquid in the logarithmic growth phase, centrifuge and wash the cells twice with 5mL buffer solution at 3°C. The composition of the buffer solution is as follows: 0.01M Tris (pH7.8), 0.017M magnesium acetate and 0.06M potassium chloride. The resulting cells were frozen at -70°C, and after thawing, they were ground for 15 minutes with aluminum oxide twice the wet weight of the cells to obtain a crude extract of S30 ribosomes. Dissolve the crude extract of S30 ribosomes in 0.25mL of 0.017M magnesium acetate buffer, add a certain concentration of the test compound, incubate at room temperature for 15min, and then add primer polyuridine, 4×10 -9 mol[ 14 C] phenylalanine, 5 × 10 -9 mol of phenylalanine and 5 x 10 -9 mol of other essential amino acids, continue to incubate for 15 min. The synthesized protein was precipitated by adding 1 mL of 10% trichloroacetic acid solution at 3 °C, f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com