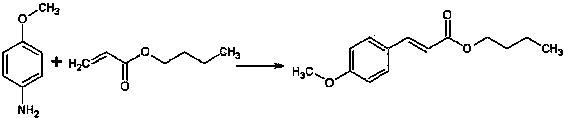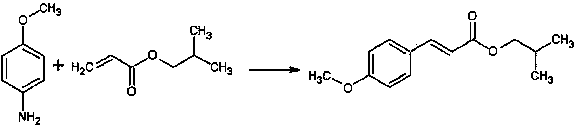P-methoxy cinnamic acid ester preparation method
A technology for methoxymeat and p-methoxyaniline, which is applied in the field of preparing p-methoxycinnamate, can solve the problems of catalyst regeneration, high process cost, low total yield and the like, achieves low pollution and simple operation process , the effect of saving cooling costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
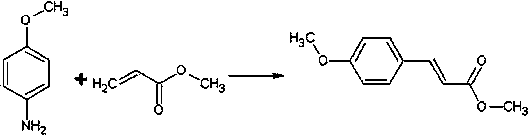
[0018] A method for preparing methyl p-methoxycinnamate, the steps comprising:
[0019]
[0020] 1) Mix 1 mol (123g) of p-methoxyaniline with 553g of 75wt% sulfuric acid solution, control the temperature not to exceed 50°C (30-50°C), and add dropwise 25wt% sodium fluoroborate solution (containing 56g sodium fluoroborate);
[0021] 2) After dropping, add 1.2mol (103g) methyl acrylate, 0.8g dodecyltrimethylammonium bromide and catalyst (5.5g copper oxide, 8.9g iron oxide, 8.5g zinc oxide), and stir well;
[0022] 3) Add 21wt% sodium nitrite solution (containing 75.8g sodium nitrite) dropwise while stirring, and finish dropping in 60 minutes. During the dropping process, control the internal temperature at 30-45°C. After dropping, keep the temperature and continue the reaction for 40 minutes. minute;
[0023] 4) Raise the temperature to 60°C and stop the reaction after 1.5 h of reaction.
[0024] After the reaction was completed, dilute with about the same volume of water a...
Embodiment 2
[0026] A method for preparing butyl p-methoxycinnamate, the steps comprising:
[0027]
[0028] 1) Mix 1 mol (123g) of p-methoxyaniline with 394g of 60wt% sulfuric acid solution, control the temperature not to exceed 50°C (30-50°C), and add dropwise 18wt% sodium fluoroborate solution (containing 31g sodium fluoroborate);
[0029] 2) After dropping, add 1.05mol (134.6g) of n-butyl acrylate, 0.7g of tetrabutylammonium bromide and catalyst (1.6g of copper oxide, 1.3g of iron oxide, 1.47g of zinc oxide), and stir well;
[0030] 3) Cool down to 30°C, add dropwise a 19wt% sodium nitrite solution (containing 71g of sodium nitrite) while stirring, and finish dropping in 30 minutes. During the dropping process, control the internal temperature to 30-45°C. Temperature continued to react for 30 minutes;
[0031] 4) Raise the temperature to 50°C and stop the reaction after 1 h of reaction.
[0032] After the reaction, dilute with about the same volume of water as the reaction soluti...
Embodiment 3
[0034] A method for preparing isobutyl p-methoxycinnamate, the steps comprising:
[0035]
[0036] 1) Mix 1 mol (123g) of p-methoxyaniline with 500g of 70wt% sulfuric acid solution, cool down to 40°C, add dropwise 20wt% sodium fluoroborate solution (containing 50g sodium fluoroborate), dropwise Keep the temperature below 50°C during the process;
[0037] 2) After dropping, add 1.1mol (140g) isobutyl acrylate, 1g tetrabutylammonium chloride and catalyst (copper oxide 3.9g, iron oxide 4.8g, zinc oxide 4.9g), stir well;
[0038] 3) Cool down to 40°C, add dropwise a 20wt% sodium nitrite solution (containing 74.5g of sodium nitrite) while stirring, and finish dropping in 50 minutes. During the dropping process, control the internal temperature to 30-45°C. Maintain temperature and continue to react for 35 minutes;
[0039] 4) Raise the temperature to 55°C and stop the reaction after 1.5 h of reaction.
[0040] After the reaction, dilute with about the same volume of water as t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com