DNA (deoxyribonucleic acid) vaccine of HCV (hepatitis C virus) and preparation method thereof
A DNA vaccine and envelope glycoprotein technology, which is applied in the fields of molecular biology and infection immunity, can solve the problems of low immune protection of HCVE2 protein, difficulty in purification, difficulty, etc., achieves neutralization of further infection by virus, overcomes difficulties, and has a simple method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Construction of eukaryotic recombinant plasmids for E2 mutants with secretory glycosylation single-point deletion mutations
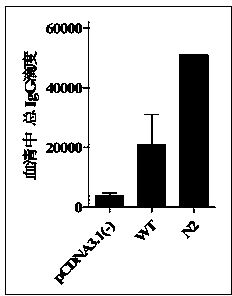
[0029]The E2 gene sequence that can express the secreted E2 protein (Secreted E2, sE2) was amplified from the HCV type 1a genome sequence (GenBank Acc.No.AY958062) by PCR, and at the same time, it started at the N-terminal of the sequence The upstream of the codon (ATG) introduced a bacterial-derived non-methylated CpG sequence (GACGTT), and further constructed the fragment into the eukaryotic expression vector pcDNA3.1(-) (Invitrogen). Then, the asparagine at the second N-glycosylation site (N423RT) on sE2 was mutated to aspartic acid (D423RT) by the PCR site-directed mutagenesis method, and the recombinant plasmids obtained were named PingN2.
[0030] The primers used in this example are shown in the table below:
[0031]
[0032] (The italics in the table indicate the restriction site, the underlined site is the non-methylated CpG sequence...
Embodiment 2
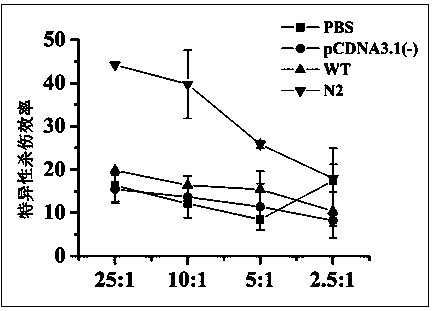
[0041] Material preparation and mouse immunization process
[0042] The endotoxin-free plasmid DNA extraction kit (Endo Free Plasmid MaxiKit) from OMEGA Biotek was used to obtain the recombinant sE2 wild-type plasmid pcDNA3.1-sE2 (WT) and the N-glycosylation site mutant plasmid N2, Perform concentration determination and adjust the plasmid concentration of each group to 2 μg / μL. Further use Ao reagent (Zhanjiang Andus Biological Co., Ltd.) to test the residual endotoxin in the obtained recombinant plasmids. The test results show that the endotoxin content in the obtained plasmid DNA is less than 0.06Edotoxin Unit / μg DNA, which meets the requirements of plasmid DNA products for gene therapy. Bacterial endotoxin residue standard (less than 0.1EU / μg).
[0043] Preparation of prokaryotic expression sE2 protein: The 384-661 bases on the HCV genome were constructed into the prokaryotic expression vector pET28a vector (Invitrogen) using molecular cloning technology, and the expresse...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com