DNA (Deoxyribose Nucleic Acid) vaccine of HCV (Hepatitis C Virus) and preparation method thereof
A nucleic acid vaccine and mutant technology, applied in the fields of molecular biology and infection immunity, can solve the problems of low eukaryotic expression yield, low E1E2 immune protection, difficult purification, etc., and achieve the effect of strong immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
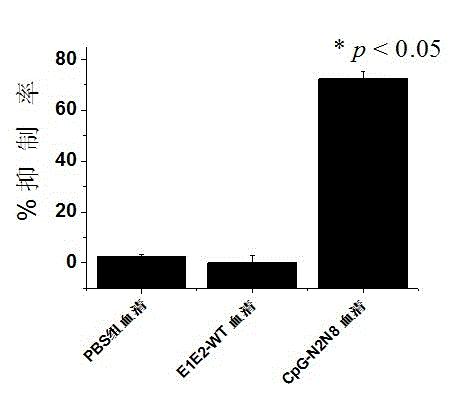
Image
Examples
Embodiment 1
[0022] 1. Construction of eukaryotic recombinant plasmids containing E1E2 wild type and mutants
[0023] The vector is pVAX-1, pcDNA3.1-E1E2 plasmid vector (Chen F, et al., Antimicrobial agents and Chemotherapy, 2010, p3355-3364) containing the full-length gene sequence of E1E2 of complete type 1a HCV (gene sequence number is AY958062 ), digested with BamHI+EcoRI and inserted into pVAX-1 (Invitrogen) vector. . The asparagine at the corresponding glycosylation site on E1E2 was mutated into aspartic acid by site-directed mutagenesis PCR. BamHI+EcoRI double digested pVAX-1, and E1E2 and glycosylation mutant fragments, ligated, and obtained recombinant plasmids named flat pVAX-CpG-N2N8, CpG-N8, CpG-N13, CpG-N2N4, CpG-N2N8 , CpG-N2N14. The primers required for construction are as follows:
[0024] E1E2-P1:
[0025] 5'-CGGGATCCGCCACCATGGGTTCCTCTTTTTTCTAT
[0026] E1E2-P2:
[0027] 5'-CGGAATTCTACGCCTCCGCTTGGGATA
[0028] E1E2-P3:
[0029] 5’-CAATACTCGAGTCAGGGCAATCAT
[0030...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com