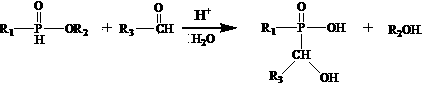Preparation method of monohydroxy dialkyl phosphinic acid metal salt fire retardant
A technology of monohydroxydialkylphosphinic acid and monohydroxyalkylphosphinic acid, which is applied in the field of preparation of monohydroxydialkylphosphinic acid metal salt flame retardants, to improve safety, novel synthesis route, and simplify production The effect of equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] A preparation method of monohydroxy dialkylphosphinic acid metal salt flame retardant, comprising the following steps:
[0023] 1) Reaction of alkyl phosphine dichloride with alcohol to obtain mono-alkyl phosphonate;
[0024] 2) In the presence of acid and water, react the monoalkylphosphonate obtained in the previous step with aldehyde to obtain a monohydroxyalkylphosphinate solution;
[0025] 3) React the monohydroxyalkyl hypophosphite solution obtained in the previous step with Lewis acid in water to form a solid monohydroxydialkylphosphinate metal salt flame retardant, which can be separated, washed and dried.
[0026] In step 1), the alkyl phosphine dichloride is methyl phosphine dichloride, ethyl phosphine dichloride, propyl phosphine dichloride, phenyl phosphine dichloride, isopropyl phosphine dichloride , One of cyclohexylphosphine dichloride.
[0027] In step 1), the alcohol is one of methanol, ethanol, propanol, isopropanol, butanol, isobutanol, octanol, iso...
Embodiment 1
[0045] 1) Synthesis of monoethyl methylphosphite: Dissolve 1.0mol of ethanol and 0.5mol of N,N-xylaniline in 5-6 times the volume of n-hexane at 0-5°C (n-hexane The volume is 5-6 times the total volume of ethanol and N, N-xylidine), under stirring, add 0.5 mol of dichloromethyl phosphine dropwise, keep stirring at this temperature for 1.5 hours, rise to 20-25°C and continue Stir for 1.5 hours, then reflux for 1.5 hours. Cool to room temperature, filter, wash the filter cake with acetone, and use the filtrate in the next step after removing the solvent.
[0046] 2) Add 100ml of deionized water, 45ml of 36wt% hydrochloric acid and 15g of paraformaldehyde to the solvent-free filtrate in 1), stir and heat to 60°C for 15 hours to obtain 195g of monohydroxymethyl hypophosphorous acid solution, liquid phase Chromatographic analysis contained 25.6% of monohydroxymethyl hypophosphorous acid (molecular weight M=110), and the yield was 90%.
[0047] 3) Maintain the temperature at 60°C ...
Embodiment 2
[0050]1) Dissolve 1.0mol of propanol and 0.5mol of N,N-xylidine in 5-6 times the volume of n-hexane at 0-5°C, and add 0.5mol of dichloromethyl dropwise under rapid stirring Phosphine, keep stirring at this temperature for 1.0 hour, rise to 20-25°C and continue stirring for 1.5 hours, then reflux for 2 hours. Cool to room temperature, filter, wash the filter cake with acetone, and use the filtrate in the next step after removing the solvent.
[0051] 2) Add 100ml of deionized water, 45ml of 36wt% hydrochloric acid and 15g of paraformaldehyde to the solvent-free filtrate in 1), stir and heat to 65°C for 13 hours to obtain 201g of monohydroxymethyl hypophosphorous acid solution, liquid phase Chromatographic analysis contained 25.4% of monohydroxymethylphosphorous acid (M=110), yield 92%.
[0052] 3) Maintain the temperature at 65°C and add an aqueous solution prepared by adding 21g of aluminum chloride and 50ml of deionized water to the monohydroxymethyl hypophosphorous acid sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com