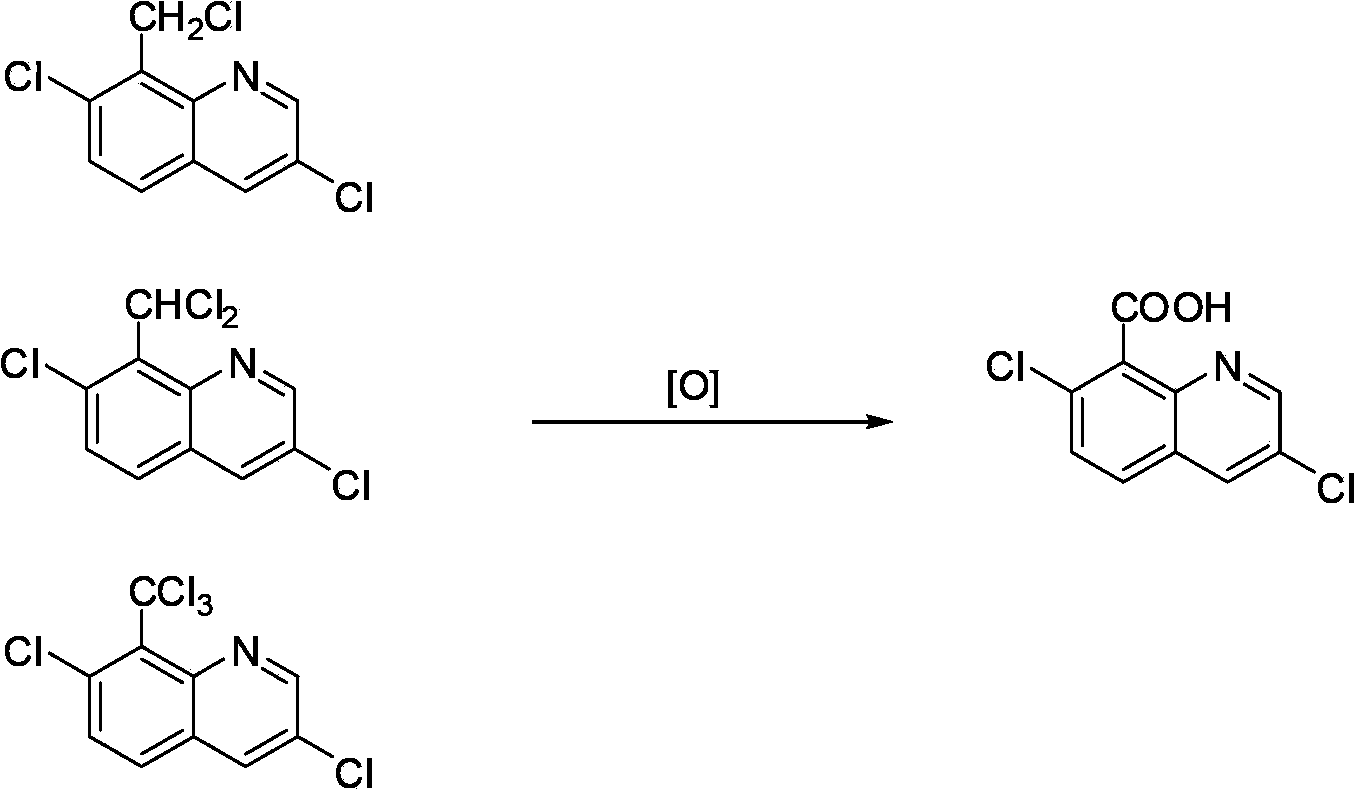
Liquid-phase catalytic oxidation quinclorac preparation method
A technology of quinclorac and liquid-phase catalysis, which is applied in the field of preparation of quinclorac by liquid-phase catalytic oxidation, can solve the problems of large amount of waste acid, low product purity, and difficulty in handling, etc., so as to reduce the discharge of waste liquid, The post-treatment process is simplified and the effect of less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add the reaction liquid to a titanium autoclave with a volume of 0.5L, feed oxygen to a pressure of 1.0Mpa, and heat the reaction liquid to 150°C and pressure to 1.6Mpa while stirring. The composition of the reaction solution (i.e. the reaction mixture) is 80.0g of 7-chloro-8-methylquinoline chloride and 300.0g of acetic acid, 5.7g of cobalt acetate tetrahydrate, 1.8g of manganese acetate tetrahydrate, 4.9g 40% of hydrogen bromide. After 4.1 hours of reaction, the reaction system no longer consumes oxygen, and the reaction ends. After cooling down to room temperature and discharging, the solid product was separated and dried. Obtain quinclorac dry product 75.0g, content 86.8%.
[0021] Add 200.0 g of absolute ethanol to 75.0 g of quinclorac crude product, stir and heat up to reflux, keep warm for 2 hours, cool to below 10°C, filter, distill the filtrate to recover ethanol for mechanical use, and dry the filter cake to obtain 63.8 g of quinclorac fine product, The con...
Embodiment 2
[0023] The mother liquor after the solid product was separated in Example 1 was removed through the acetic acid solution distilled out under reduced pressure to remove the water contained therein to obtain 264.0 g of acetic acid.
[0024] Add the reaction liquid to a titanium autoclave with a volume of 0.5L, feed oxygen to a pressure of 1.0Mpa, and heat the reaction liquid to 150°C and pressure to 1.6Mpa while stirring. The composition of the reaction solution (i.e. the reaction mixture) is 80.0 g of 7-chloro-8-methylquinoline chloride, 264.0 g of reclaimed acetic acid, 36.0 g of fresh acetic acid, 1.9 g of cobalt acetate tetrahydrate, 1.8 g of Manganese acetate hydrate, 8.9g of 40% hydrogen bromide. After 4.2 hours of reaction, the reaction system no longer consumes oxygen, and the reaction ends. After cooling down to room temperature and discharging, the solid product was separated and dried. Obtain quinclorac dry product 75.5g, content 86.4%.
[0025] Add 200.0g of absol...
Embodiment 3~10
[0027] Reaction conditions, operation steps and mother liquor treatment recovery mode are the same as embodiment 2. Catalyst consumption and experimental results are shown in Table 1 in each embodiment.
[0028] Table 1 Liquid phase catalytic oxidation prepares quinclorac
[0029] Example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com