Recombinant or transgenic factor VII compound having a majority of glycan, biantennary, bisialylated and non-fucosylated forms
A technology of non-fucosylation and sialylation, which is applied in the direction of drug combination, coagulation/fibrinolytic factors, medical preparations containing active ingredients, etc., and can solve the problem of lower efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0177] Example 1: Production of human FVII protein in milk of transgenic female rabbits
[0178] First, by introducing the WAP gene sequence (described in the document Devinoy et al, Nucleic Acids Research, vol.16, no.16, 25August1988, p.8180) into the polylinker of the p-poly III-I vector (in Plasmid p1 was prepared as described in the document Lathe et al, Gene (1987) 57, 193-201).
[0179] Plasmid p2, obtained from plasmid p1, contains the rabbit WAP gene promoter and the human FVII gene.
[0180] Transgenic female rabbits were obtained by traditional microinjection technique (Brinster et al, Proc. Natl. Acad. Sci. USA (1985) 82, 4438-4442). 1-2p1, which contains 500 copies of the gene, was injected into the male pronuclei of mouse embryos. The Not 1-Not 1 fragment of this plasmid containing the recombinant gene was microinjected. Afterwards, the embryos are transferred into the fallopian tubes of hormone-prepared succession females. About 10 percent of the manipulated ...
Embodiment 2
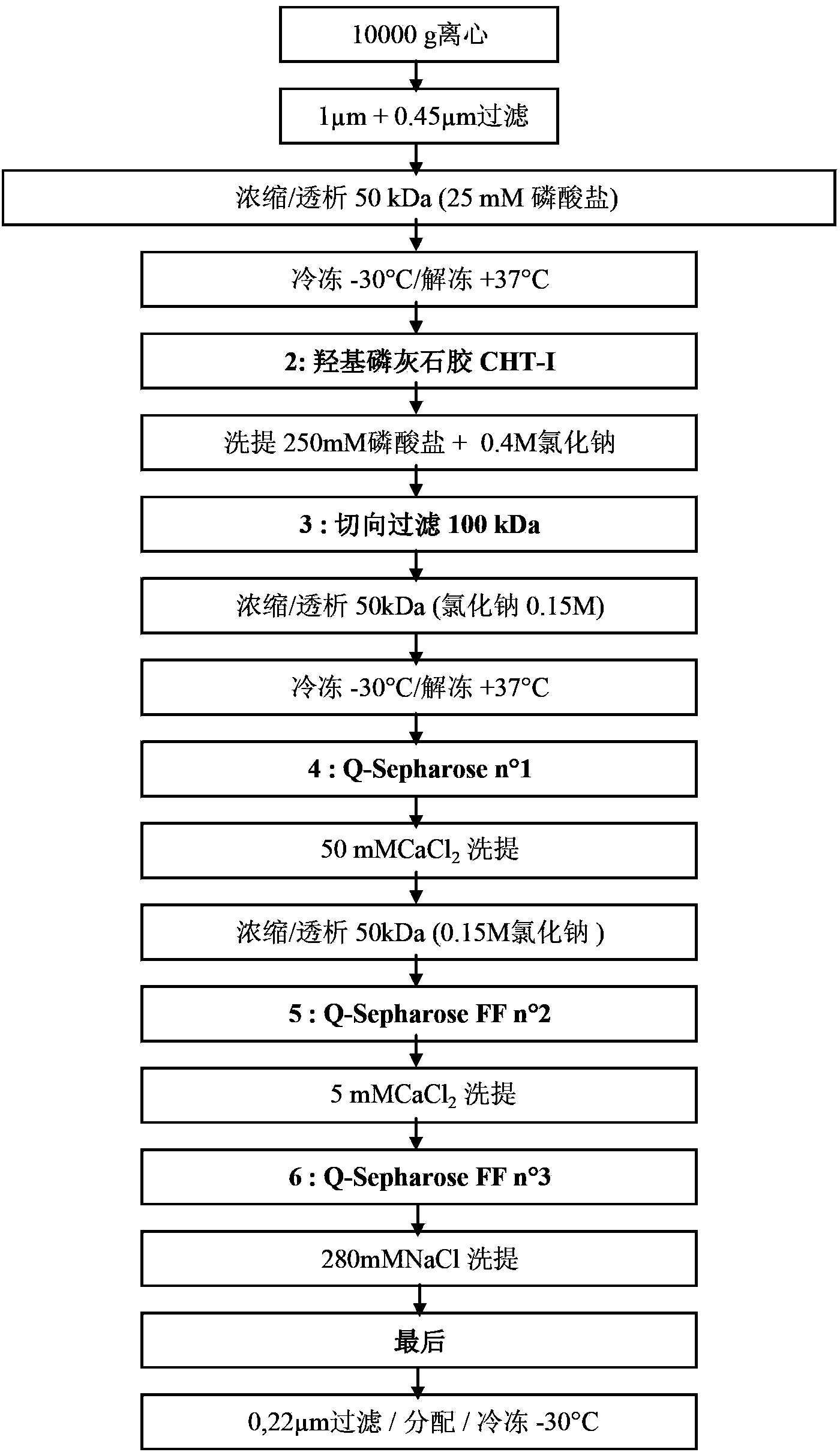
[0182] Example 2: Extraction and purification of obtained FVII
[0183] a) Extraction of FVII
[0184] Take 500ml whole raw milk and dilute it with 9 times the volume of 0.25M sodium phosphate with pH8.2. After stirring at room temperature for 30 minutes, the FVII-rich aqueous phase was centrifuged at 10000 g for 1 hour at 15° C. (centrifuge Sorvall Evolution RC-6700 rev / min-rotor SLC-6000). 6 cans of about 835ml are required.
[0185] After centrifugation, three phases are present: a lipid phase at the surface (milk fat), a clear FVII-rich aqueous non-lipid phase (major phase) and a remaining white solid phase (precipitated insoluble casein and calcium compounds).
[0186] The FVII-enriched aqueous non-lipid phase was collected by a peristaltic pump until the lipid phase. The lipid phase is recovered separately. The solid phase (precipitate) was removed.
[0187] However, the aqueous non-lipid phase still contained a very small amount of lipid, which was filtered through...
Embodiment 3
[0233] Example 3: Characterization of Glycosylation Sites and Glycopeptides by MS-ESI
[0234] The N-glycosylation sites of FVII-Tg, FVIIa,p (plasma FVII) and FVIIa,r were identified by LC-ESIMS( / MS), confirmed by MALDI-TOFMS, and each site was analyzed by LC-ESIMS The relative proportions of different glycans were determined.
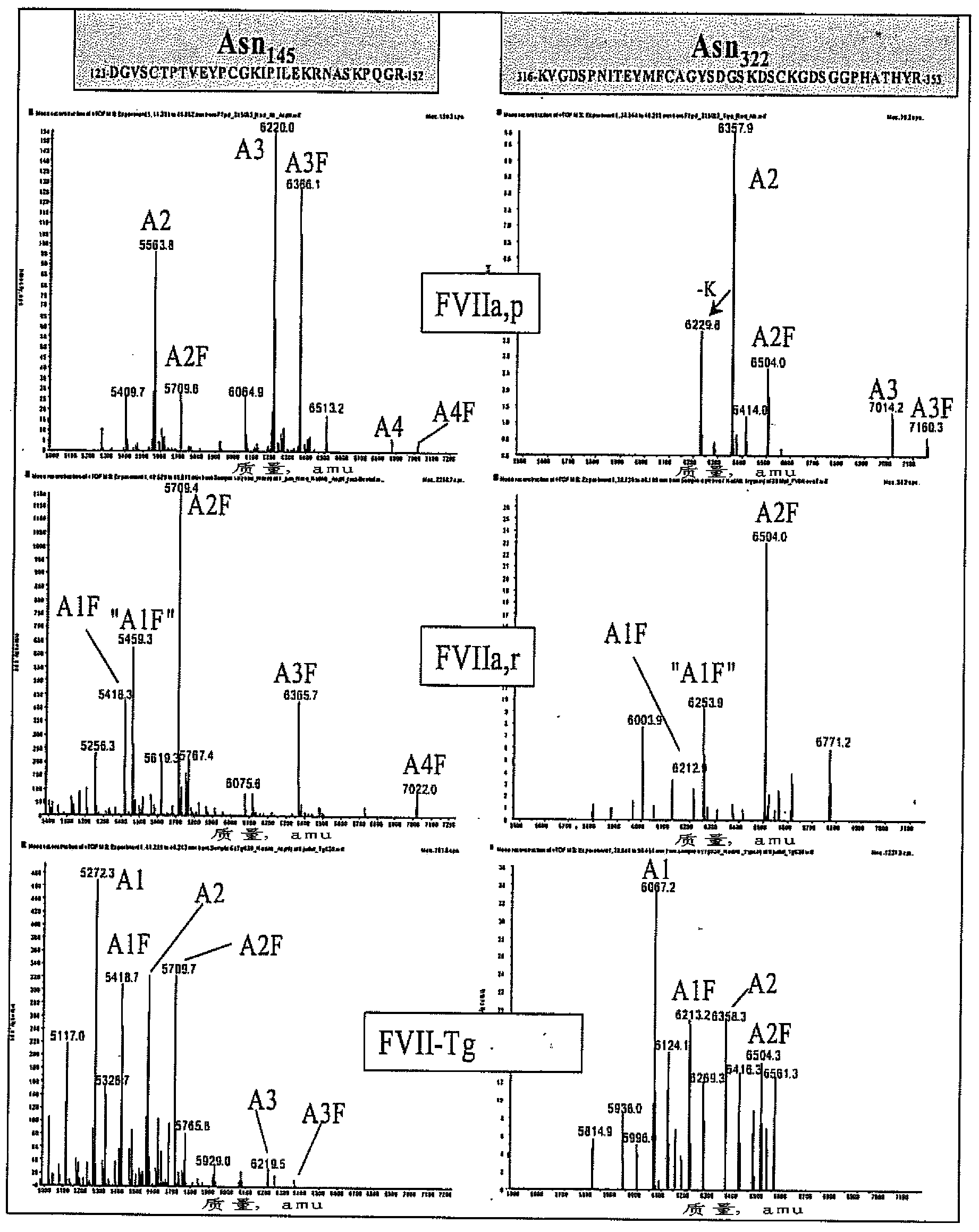
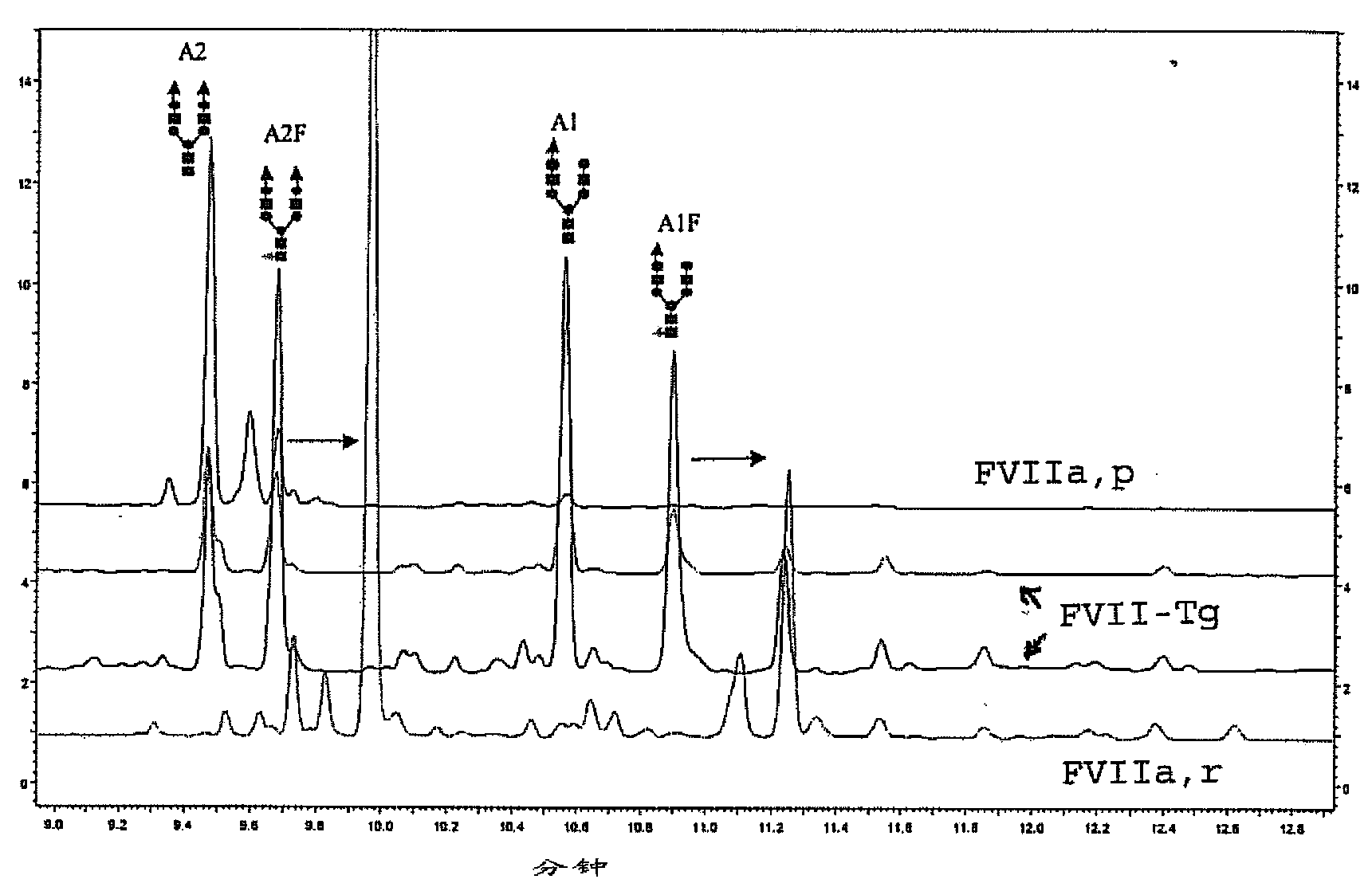
[0235] figure 2 Deconvoluted ESI spectra of glycopeptides containing two glycosylated asparagine residues are depicted. The positions of glycosylation sites were confirmed by MALDI-TOF ( / TOF) and Edman sequencing.
[0236] Pairs exhibiting N-glycosylation site Asn 145 and Asn 322 Glycopeptide of FVIIa, p [D 123 -R 152 ] and [K 31.6 -R 353 ] showed the presence of a biantennary, disialo, unfucosylated structure (A2) (the observed Asn-containing 145 glycopeptide mass: 5563.8 Da) and the fucosylated structure (A2F) (observed glycopeptide mass with Asn145: 5709.8 Da). Note also that Asn 145 Triantennary, trisialyl, unfucosylated (A3) (observed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com