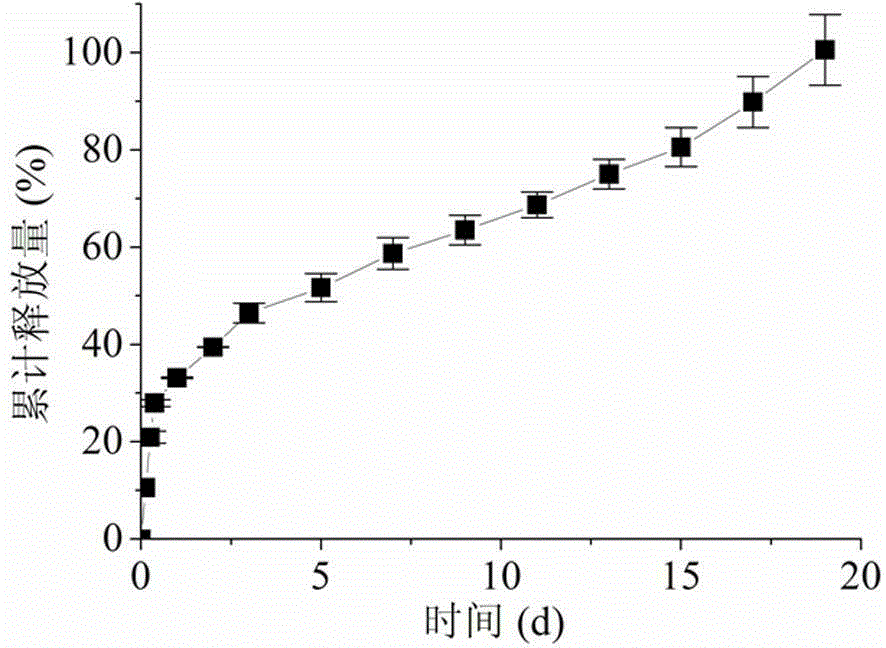
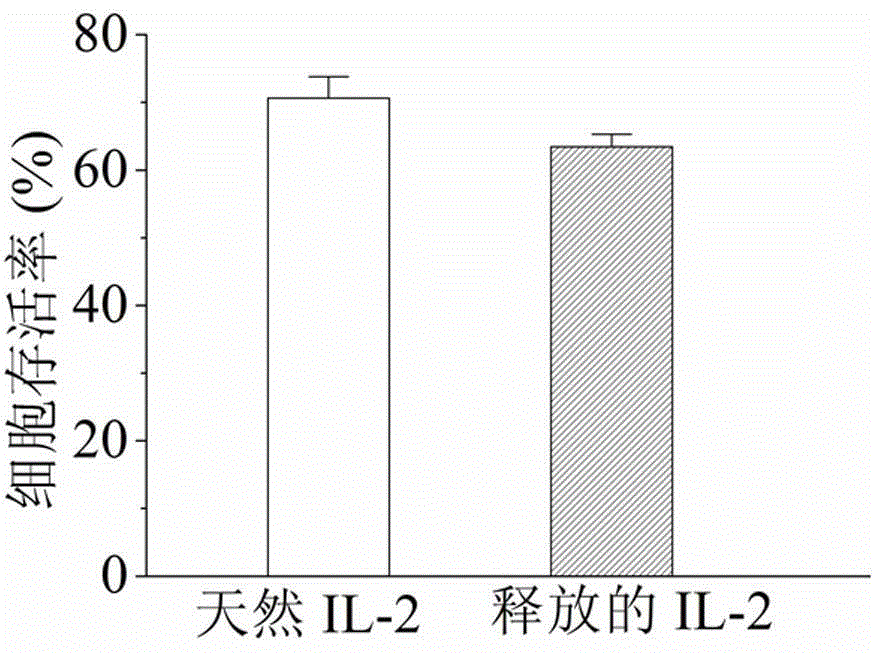
Hydrogel, preparation method thereof and applications
A hydrogel and buffer technology, applied in medical science, prosthesis, etc., can solve the problems of many synthesis steps of ring tension alkynyl group, low storage modulus of hydrogel, long gel time, etc., and achieve excellent biological activity. , excellent fluorescence performance, simple preparation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment one: four-arm polyethylene glycol tetrazole derivatives (PEG-TET 4 )Synthesis
[0050] (1) In a 100mL two-necked bottle, under nitrogen protection, take 0.75 g (5 mmol) of p-formylbenzoic acid and dissolve it in 50 mL of ethanol, fully dissolve, add 0.86 g (5 mmol) of benzenesulfonyl hydrazide, dissolve , stirred for half an hour, precipitated with secondary water, and dried to obtain 1.2975 g of light yellow solid phenylhydrazone, with a yield of 85.4%. Aniline 0.23 mL (0.23 g) was dissolved in a mixed solution (2 mL water, 2 mL ethanol, 0.65 mL concentrated HCl), and NaNO 2 The solid (0.175 g) was dissolved in 1 ml of secondary water, and after dissolving, it was added dropwise to the above mixed solution and stirred for 15 min to form a light yellow diazobenzene solution. Dissolve phenylhydrazone (0.6 g) in 15 ml of pyridine, fully dissolve, add dropwise to the diazobenzene solution mentioned above, and control the reaction temperature at 0°C with an ice...
Embodiment 2
[0052] Embodiment two: chitosan tetrazole derivatives (Chit-TET n )Synthesis
[0053] Under the condition of nitrogen protection, tetrazole (0.216 g) was dissolved in DMSO (15 mL), then DCC (120 mg) was added to react for 30 min, and then added to chitosan aqueous solution (80K, 0.5 g), and At the same time, DMAP (10 mg) was added, and the mixture was stirred at room temperature for 24 hours. The polymer solution was purified by ultrafiltration and freeze-dried to obtain the product Chit-TET n (0.39 g, 78.0%). Chit-TET n NMR characterization, 1 H NMR (400 MHz, D 2 O): δ 8.15-8.21 (m, 4H, C 6 h 4 of TET); 7.47, 7.55 and 8.28 (m, 5H, C 6 h 5 of TET); 4.46 (s, 1H, the proton on the methine at the α-position of chitosan); 3.27-4.10 (m, 9H, the methylene and methine linked to the ether bond and hydroxyl group in chitosan protons on methyl groups); 2.97-3.16 (m, 1H, methine protons attached to amino groups on chitosan).
Embodiment 3
[0054] Embodiment three: four-arm polyethylene glycol methacrylate derivatives (PEG-MA 4 )Synthesis
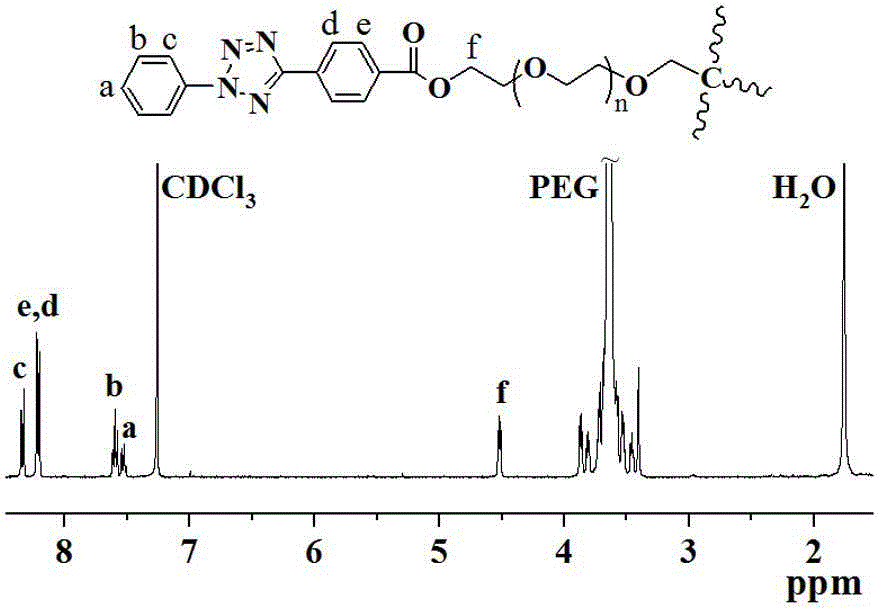
[0055] Under nitrogen protection conditions, add eight-arm polyethylene glycol PEG ( M n =10 K, 0.25g), triethylamine (Et 3 N, 120 μL), methacrylic anhydride (240 μL), DMAP (4.9 mg), toluene 10 mL, seal the reactor well, put it in a 70°C oil bath for 24 hours, then precipitate with ice ether, filter, and store at room temperature Vacuum drying to obtain product 0.45 g, yield 60.5%; PEG-4-MA NMR characterization see attached figure 2 , 1 H NMR (400 MHz, CDCl 3 ): δ 5.54 and 6.10 (s, 2H, -COC(CH 3 )CH 2 ), 4.28 (t, 2H, -COOCH 2 -), 3.61 (m, methylene protons on the PEG backbone), 1.91 (s, 3H, -COC(CH 3 )CH 2 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com