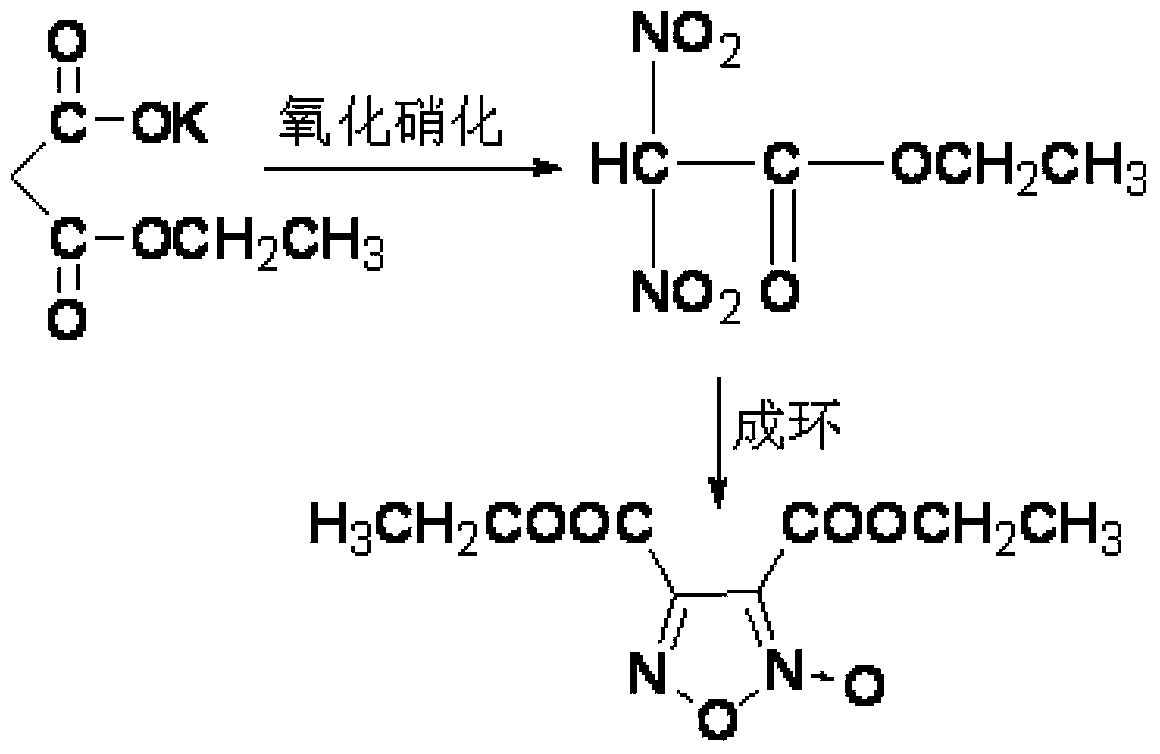
Synthesis method of 3, 4-dicarboxylic acid diethyl ester furoxan
A technology of diethyl diformate and furoxan oxide is applied in 3 fields, can solve the problems of no reported purity, low purity, and high risk of experimental operation, achieves safe and reliable synthesis process, simple and easy-to-obtain raw materials, and improved reaction yield. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] Preparation of Monoethyl Malonate Potassium Salt:
[0026] Add 50g (0.3mol) of diethyl malonate and 120mL of ethanol into the three-necked flask, heat to reflux temperature, add potassium hydroxide ethanol solution (17.5gKOH dissolved in 80ml of ethanol) dropwise, The reaction was continued for 2 hours. After cooling, a large amount of solids were precipitated, filtered, washed with alcohol, and dried to obtain 41.9 g of monoethyl malonate potassium salt, with a yield of 82.3% and a purity of 98.5%.
Embodiment 1
[0029] Add 22.1g (0.13mol) of monoethyl malonate potassium salt and 65mL of carbon tetrachloride into the three-necked flask, and dropwise add 189g (3.0mol) of industrial nitric acid with a mass concentration of 98% at 0-5°C, dropwise After the addition, 31g (0.45mol) of sodium nitrite was added in batches, the reaction temperature was controlled at 7-9°C and kept warm for 4h, the mixture was poured into ice water, the organic phase was separated, extracted with carbon tetrachloride, the organic phase was combined, and the Anhydrous MgSO 4 After drying and filtering, the filtrate was heated to reflux for 5 hours, and then the solvent was distilled off under reduced pressure to obtain a light yellow transparent liquid, 3,4-diethyl dicarboxylate furoxan, with a yield of 94.2% and a purity of 98.7% (HPLC).
[0030] Product structure identification:
[0031] IR (KBr, ν / cm-1): 1751 (C=O), 1626 (C-N), 1479 (O-N-O), 1335, 1302, 1250 (N-O), 1202 (furoxan ring), 1026, 856, 758;
[00...
Embodiment 2
[0037] Add 22.1g (0.13mol) of monoethyl malonate potassium salt and 65mL of carbon tetrachloride into the three-necked flask, and dropwise add 122.8g (1.95mol) of industrial nitric acid with a mass concentration of 98% at -5 to 0°C After the dropwise addition, 17.9 g (0.26 mol) of sodium nitrite was added, the reaction temperature was controlled at 3-6° C. and kept for 3 h, the mixture was poured into ice water, the organic phase was separated, extracted with carbon tetrachloride, and the organic phases were combined. with anhydrous MgSO 4 After drying and filtering, the filtrate was heated to reflux for 2 hours, and then the solvent was distilled off under reduced pressure to obtain a light yellow transparent liquid, 3,4-diethyl dicarboxylate furoxan, with a yield of 94% and a purity of 98.2% (HPLC).
[0038] The structure identification result of the product obtained in this embodiment is the same as that in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com