Polymethacrylate cross-linked electro-optic polymer system and synthesis method and application thereof
A technology of polymethacrylate and methacrylic acid, used in optics, nonlinear optics, instruments, etc., can solve problems such as insufficient stability, and achieve the effects of improving polarization efficiency, solubility, and electro-optic coefficient.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
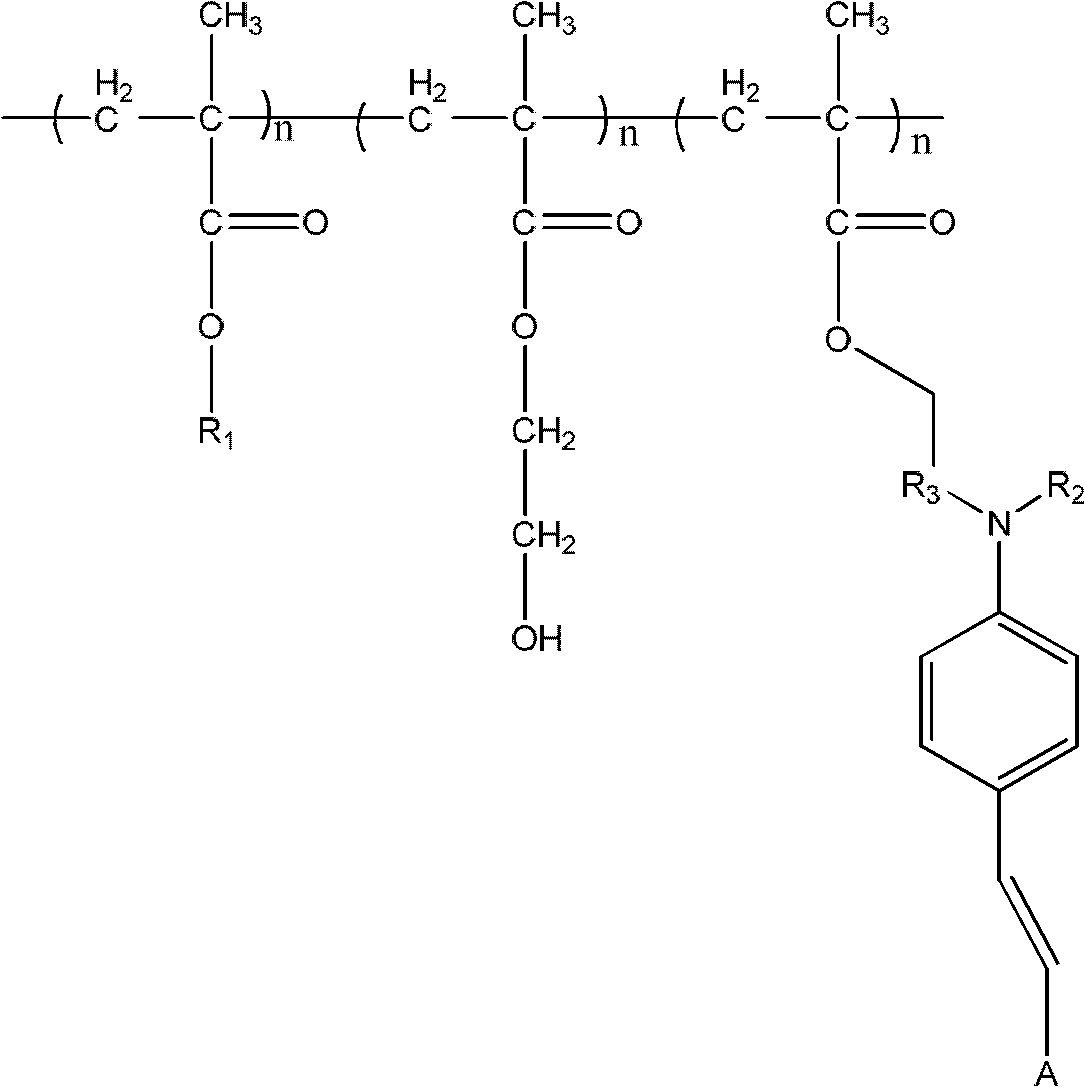
[0050] Synthesize the cross-linking system of polymethacrylate electro-optic polymer with electron-donating acceptor structure and organic second-order nonlinear optical chromophore in the side chain as shown below (n is 1-100000):
[0051]
[0052] The synthetic route is as follows:
[0053]
[0054]
[0055] The synthesis method is:
[0056] (1) Synthesis of Compound 2:
[0057] Dissolve (N-methyl, N-hydroxyethyl)-4-aminobenzaldehyde (18g, 0.1mol) in 150mL of anhydrous tetrahydrofuran, add triethylamine (12g, 0.12mol), and cool in an external ice bath to control the temperature At 20°C, a solution of methacryloyl chloride (13 g, 0.12 mol) in 50 mL of tetrahydrofuran was added dropwise, and the mixture was stirred at room temperature for 24 hours after the addition was completed. Pour it into water, extract with dichloromethane, wash the organic layer with saturated sodium bicarbonate solution, dry, spin off the solvent, and separate through column chromatography (...
Embodiment 2
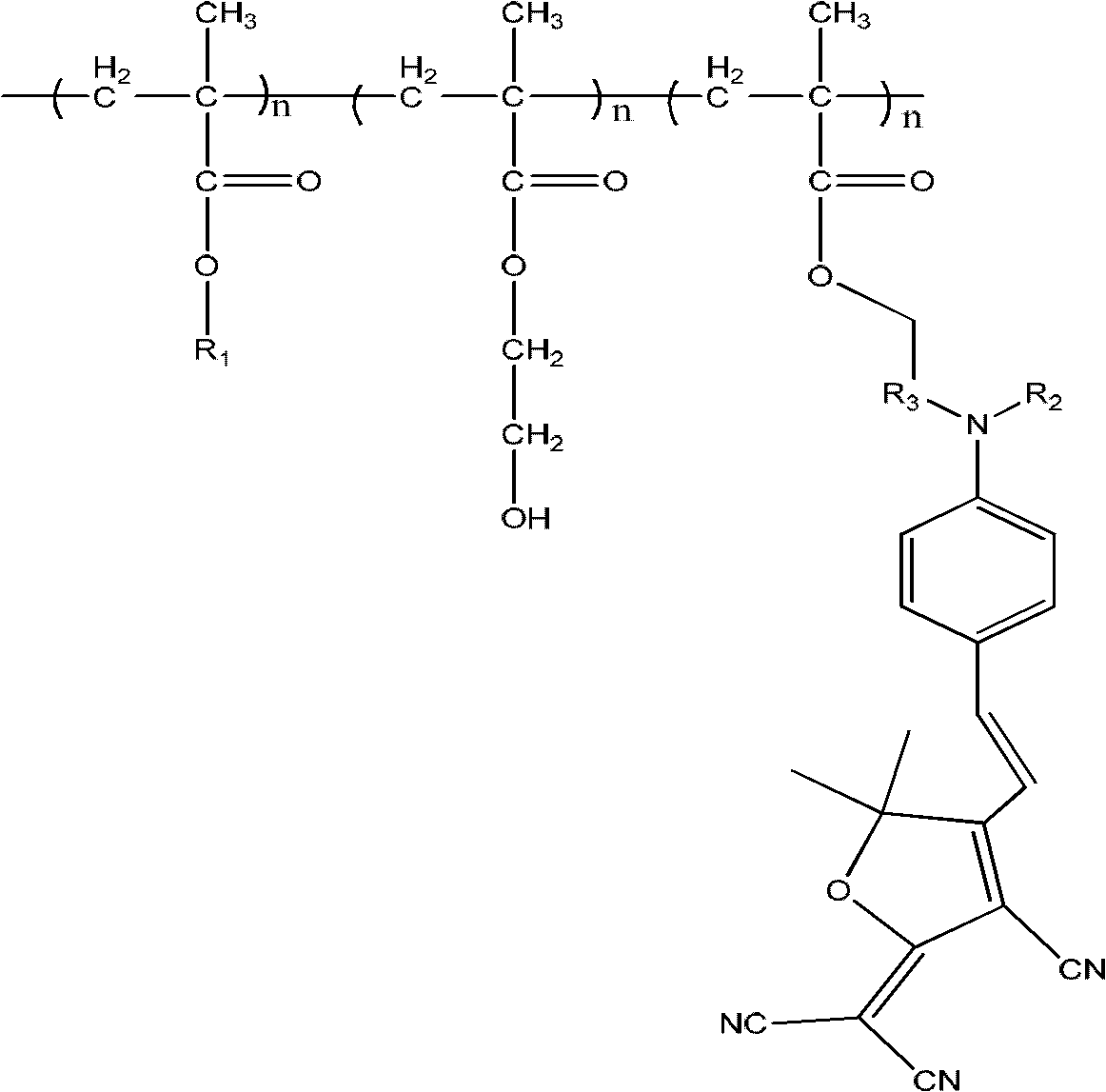
[0066] Synthesize the following polymethacrylate cross-linkable electro-optic polymer system (n is 1 ~100000):
[0067]
[0068] The synthetic route is as follows:
[0069] (1)
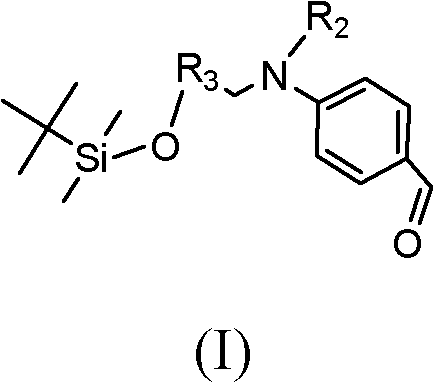
[0070]
[0071] 50g 4-(N-methyl-N-hydroxyethyl) aminobenzaldehyde (0.26mol), 50g imidazole (0.73mol), 50g tert-butyldimethylsilyl chloride (0.33mol) were dissolved in 100mL of DMF, React at room temperature for 24 hours. After the reaction was completed, the reactant was washed with saturated brine, and the washing solution was extracted with diethyl ether. After collecting the extract, it was separated and purified by column chromatography, and the eluent was petroleum ether. 50 g of compound 1 was obtained with a yield of 63%. MSm / z: 305 (M + ).
[0072] (2) The electron acceptor part is the synthesis of TCP chromophore (2)
[0073]
[0074] Dissolve 5 g of compound 1 (0.016 mol) and 5 g of tricyanopyrroline (0.027 mol) in 30 mL of ethanol, react at a temperature of 50 ° C for 45 minut...
Embodiment 3
[0096] film preparation
[0097] 0.088 g of the cross-linkable polymer synthesized in Example 1 was dissolved in 0.5 mL of cyclopentanone, stirred for 3 to 5 hours, until the cross-linkable polymer was completely dissolved, and 0.05 g of TDI (2,4- toluene diisocyanate), on the ITO glass substrate with spin coating method coating. The rotational speed was controlled to be 500-1000 rpm, and the obtained polymer film was dried in a vacuum drying oven at 60° C. for 24 hours. The thickness of the polymer film is between 1.8 and 3.5 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| electro-optic coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com