Organic electroluminescent chromophores containing electron donors and methods for their synthesis
A technology of electron donor and synthesis method, which is applied in the field of organic electro-optic chromophore and its synthesis, can solve the problem that the electro-optic coefficient is not very high, and achieve the goal of improving the macroscopic electro-optic coefficient, increasing the hyperpolarizability, and reducing the electrostatic interaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
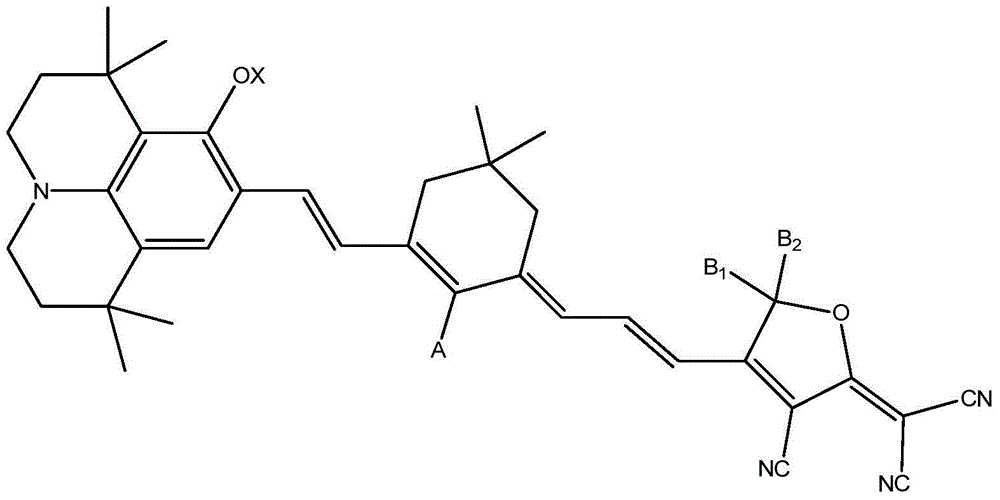
[0037] The organic electroluminescent chromophore 1 with the following structure was synthesized:
[0038]
[0039] The synthetic route is as follows:
[0040]
[0041] The synthesis method is:
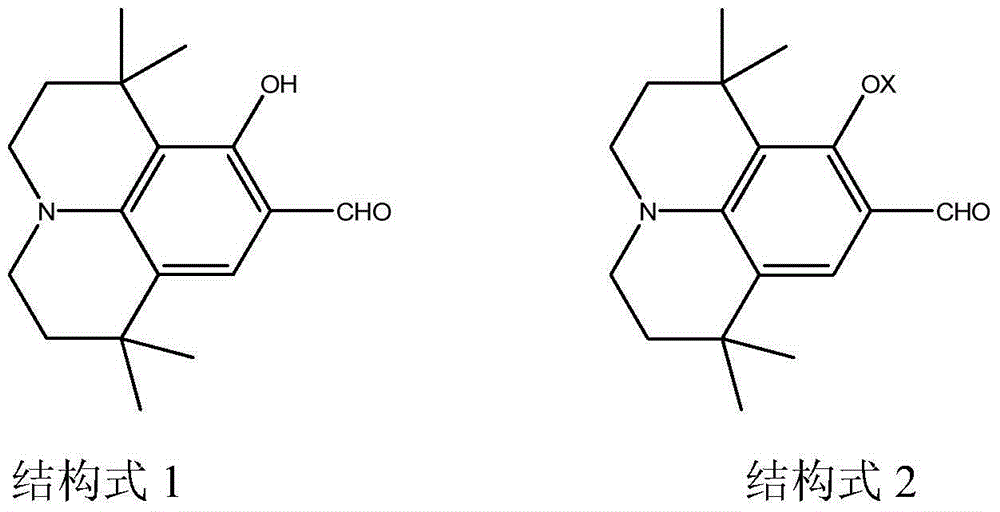
[0042] 1). Synthesis of compound 2 shown in structural formula 2
[0043] Compound 1, 1.9g n-bromobutane, 0.76g 18-crown-6 and 3g anhydrous K shown in 3.9g structural formula 1 2 CO 3 React in 80ml of DMF solution at 60°C under nitrogen atmosphere for 2 hours; after the reaction is complete, add water to the resulting product, extract with dichloromethane and evaporate dichloromethane, anhydrous MgSO 4 dry. After separation by column chromatography (adsorbent is silica gel), 4.5 g of compound 2 shown in structural formula 2 was obtained;
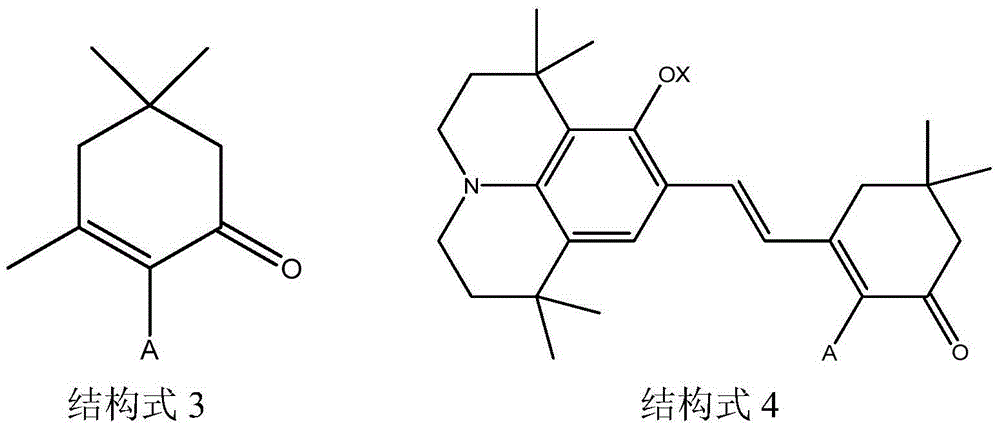
[0044] 2). Synthesis of compound 3 shown in structural formula 3
[0045] 4.5g of compound 2, 1.1g of sodium hydroxide and 1.9g of isophorone shown in structural formula 2 obtained in step 1) were stirred at room temperature for about 6 ...
Embodiment 2
[0051] The organic electroluminescent chromophore 2 with the following structure was synthesized:
[0052]
[0053] The synthetic route is as follows:
[0054]
[0055] The synthesis method is:
[0056] Basically the same as Example 1, except that the compound 4 shown in structural formula 4 obtained by 0.1 g of Example 1 step 3) and 0.08 g of SF-TCF were refluxed in 1 ml of dehydrated ethanol for 0.5 hours; dehydrated ethanol was evaporated, and column chromatography (The adsorbent is silica gel) and separated to obtain 0.1 g of the organic electroluminescent chromophore 2 product shown in the above structural formula. lambda max (CHCl 3 ):995nm; M + :854; 1 HNMR (CDCl 3 ):δ8.25(br,1H),7.59(d,2H),7.41(d,2H),7.39(s,1H),7.36(m,3H),6.91(d,1H),6.83(d, 1H),6.73(s,1H),6.44(d,1H),6.34(d,1H),3.81(t,2H),3.26(m,4H),2.45(s,2H),2.40(m,2H ),1.86(m,2H),1.73(t,4H),1.56(m,2H),1.42(s,6H),1.30(s,6H),1.03(t,3H),0.99(s,6H) .
Embodiment 3
[0058] The organic electroluminescent chromophore 3 with the following structure was synthesized:
[0059]
[0060] The synthetic route is as follows:
[0061]
[0062]
[0063] The synthesis method is:
[0064] Basically the same as Example 1, except that compound 4 and 0.066g PF-TCF shown in structural formula 4 obtained by 0.1g Example 1 step 3) were refluxed in 1ml dehydrated ethanol for 0.5 hours; steamed dehydrated ethanol, column chromatography (The adsorbent is silica gel) separation to obtain 0.11 g of the organic electroluminescent chromophore 3 product shown in the above structural formula. lambda max (CHCl 3 ):973nm; M + :772; 1 HNMR (CDCl 3 ):δ8.02(br,1H),7.49(m,5H),7.41(s,1H),7.26(d,1H),7.22(d,1H),6.38(d,1H),6.31(s, 1H), 6.26(d, 1H), 3.81(t, 2H), 3.26(m, 4H), 2.45(s, 2H), 2.30(q, 2H), 1.86(m, 2H), 1.72(t, 4H ), 1.56(m,2H), 1.42(s,6H), 1.30(s,6H), 1.02(t,3H), 0.97(s,6H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| electro-optic coefficient | aaaaa | aaaaa |
| electro-optic coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com