Neutral-amphiprotic bond-connection type second order nonlinear optics chromogen and synthesizing process thereof
A technology of second-order nonlinearity and synthesis method, which is applied in the field of neutral-amphoteric bonding type second-order nonlinear optical chromophore and its synthesis, can solve the problem of reducing the macroscopic nonlinear optical coefficient of organic materials, etc., and achieves the improvement of macroscopic Second-order nonlinear optical coefficient, large hyperpolarizability, and the effect of reducing molecular agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
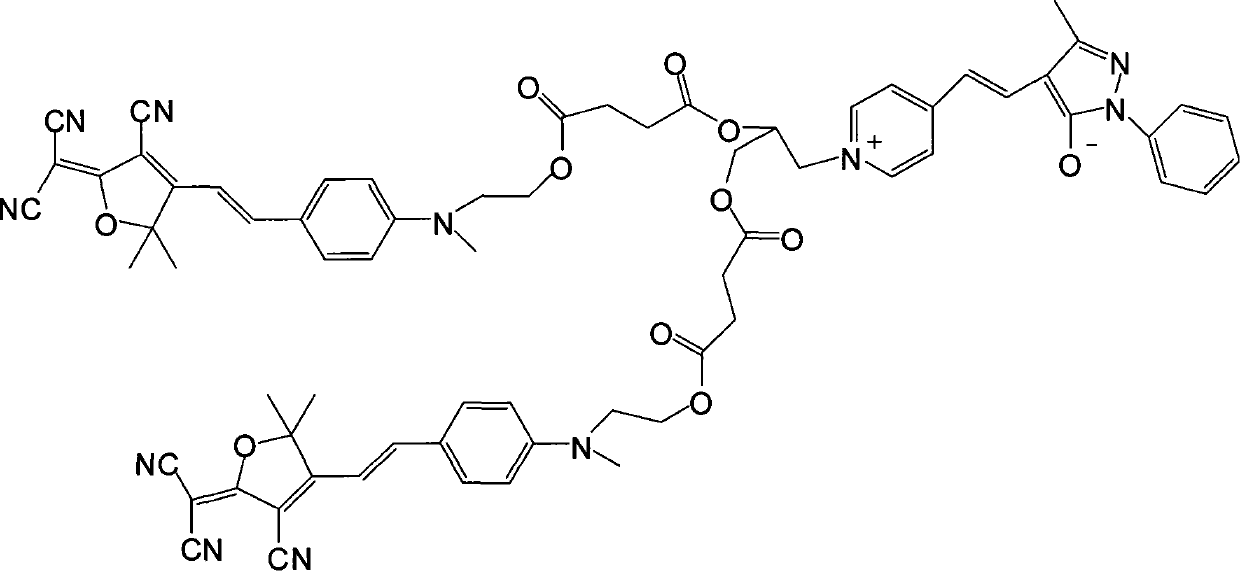
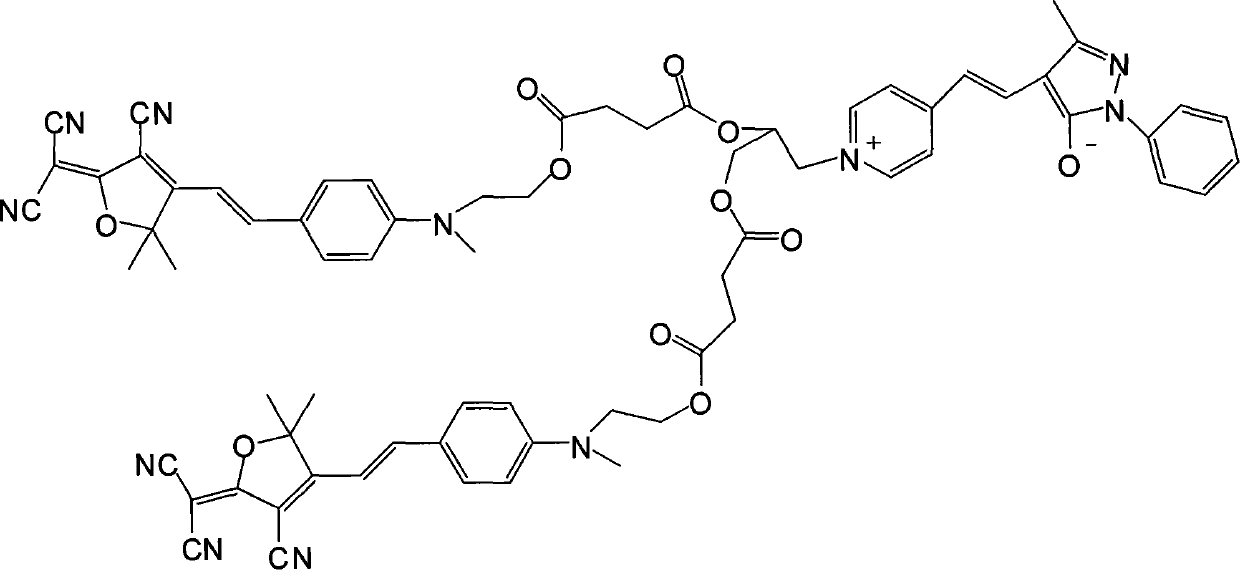
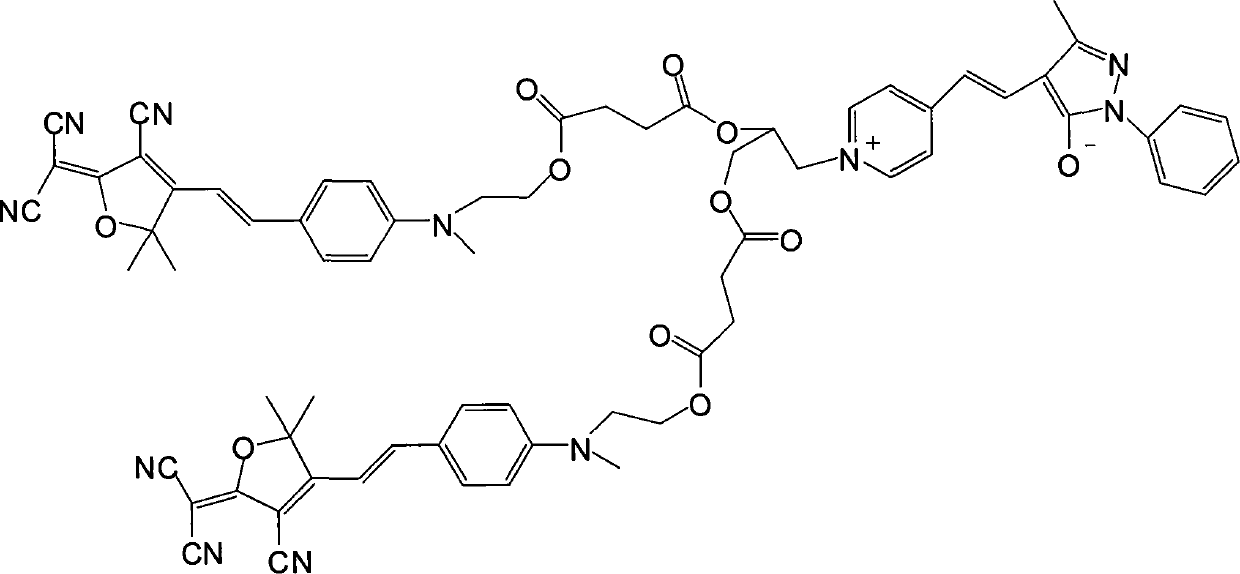
[0022] (1) Dissolve 1 mole of methyl hydroxyethyl benzaldehyde and 1 mole of tricyanofuran in 300 ml of ethanol, add 1 ml of piperidine for catalysis, and react under reflux for 6 hours. The precipitate was filtered and dried in vacuo to obtain dark blue (E)-2-(3-cyano-4-(4-((2-hydroxyethyl)(methyl)amino)styryl)-5,5-di Methylfuran-2(5H)-divinylide) propanedicyanide. Yield 67%.
[0023] Elemental analysis: Anal.Calcd for C 21 h 20 N 4 o 2 (360.41): C, 69.98; H, 5.59; N, 15.55. Found: C, 69.76; H, 5.55; N, 15.43.
[0024] Decomposition temperature: 278.61°C.
[0025] Its synthetic reaction formula is:
[0026]
[0027] (2) Get 1 mole of the product obtained in step 1) and 1.2 moles of succinic anhydride and dissolve them in 600ml of dichloromethane, add 6ml of pyridine and 0.2 moles of p-dimethylaminopyridine as a catalyst, stir the reaction overnight at room temperature, and the reaction ends Pour it into water, then wash with salt water and deionized water respectiv...
Embodiment 2
[0044] (1) Dissolve 1 mole of methyl hydroxyethyl benzaldehyde and 1.2 moles of tricyanofuran in 300 ml of ethanol, add 1 ml of piperidine to catalyze, and react under reflux for 6 hours. The precipitate was filtered and dried in vacuo to obtain dark blue (E)-2-(3-cyano-4-(4-((2-hydroxyethyl)(methyl)amino)styryl)-5,5-di Methylfuran-2(5H)-divinylide) propanedicyanide. Yield 69%.
[0045] Elemental analysis: Anal.Calcd for C 21 h 20 N 4 o 2 (360.41): C, 69.98; H, 5.59; N, 15.55. Found: C, 69.76; H, 5.55; N, 15.43.
[0046] Decomposition temperature: 278.61°C.
[0047] (2) Get 1 mole of the product obtained in step 1) and 1.5 moles of succinic anhydride and dissolve it in 600ml of methylene chloride, add 2ml of triethylamine and 0.2 mole of p-dimethylaminopyridine as a catalyst, and stir the reaction at room temperature overnight, After the reaction, pour it into water, then wash it with brine and deionized water respectively, extract the organic layer and dry it with anhy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com