Related protein capable of adjusting translation efficiency of chloroplast protein and improving heat resistance of plants, and application thereof
A chloroplast and plant technology, applied in the fields of botany and biology, can solve the problems of undiscovered chloroplast protein translation efficiency, plant heat-resistant thylakoid membrane heat-resistant proteins, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
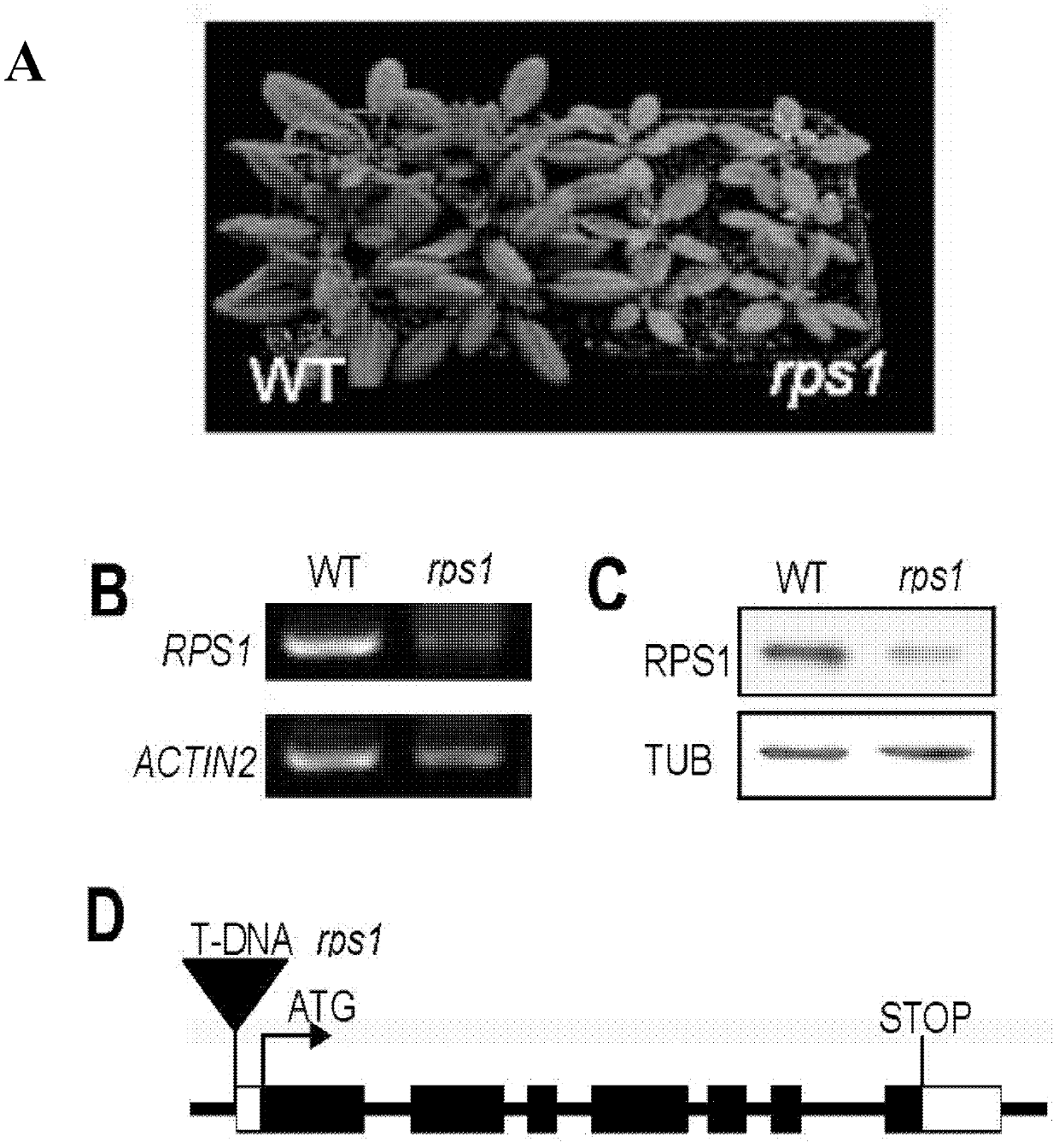
[0087] Identification of rps1 T-DNA mutants
[0088] The inventor purchased the rps1 T-DNA mutant from the Salk website, and in the current planting period, the rps1 T-DNA mutant isolated plants with yellow leaves. According to Salk's prediction, the T-DNA insertion site of rps1 is in the first exon of the RPS1 gene.
[0089] Based on the sequence information, the inventors synthesized the identification primer sequence of RPS1 (CS874869) T-DNA insertion mutant:
[0090] LP(874869-L): 5'-GACTCCAGCTGGTTTAGAGGG-3' (SEQ ID NO.: 4);
[0091] RP(874869-R): 5'-CAAGTAAGCCGATGACTTTGC-3' (SEQ ID NO.: 5);
[0092] LB1: 5'-GCCTTTTCAGAAATGGATAAATAGCCTTGCTTCC-3' (SEQ ID NO.: 6); and
[0093] LB2: 5'-GCTTCCTATTATATCTTCCCAAATTACCAATACA-3' (SEQ ID NO.: 7).
[0094] Use the above primers as a primer pair, and use the genomic DNA of the rps1 T-DNA mutant as a template to perform a PCR reaction to identify the T-DNA insertion position in the rps1 mutant (the PCR product is connected to the T...
Embodiment 2
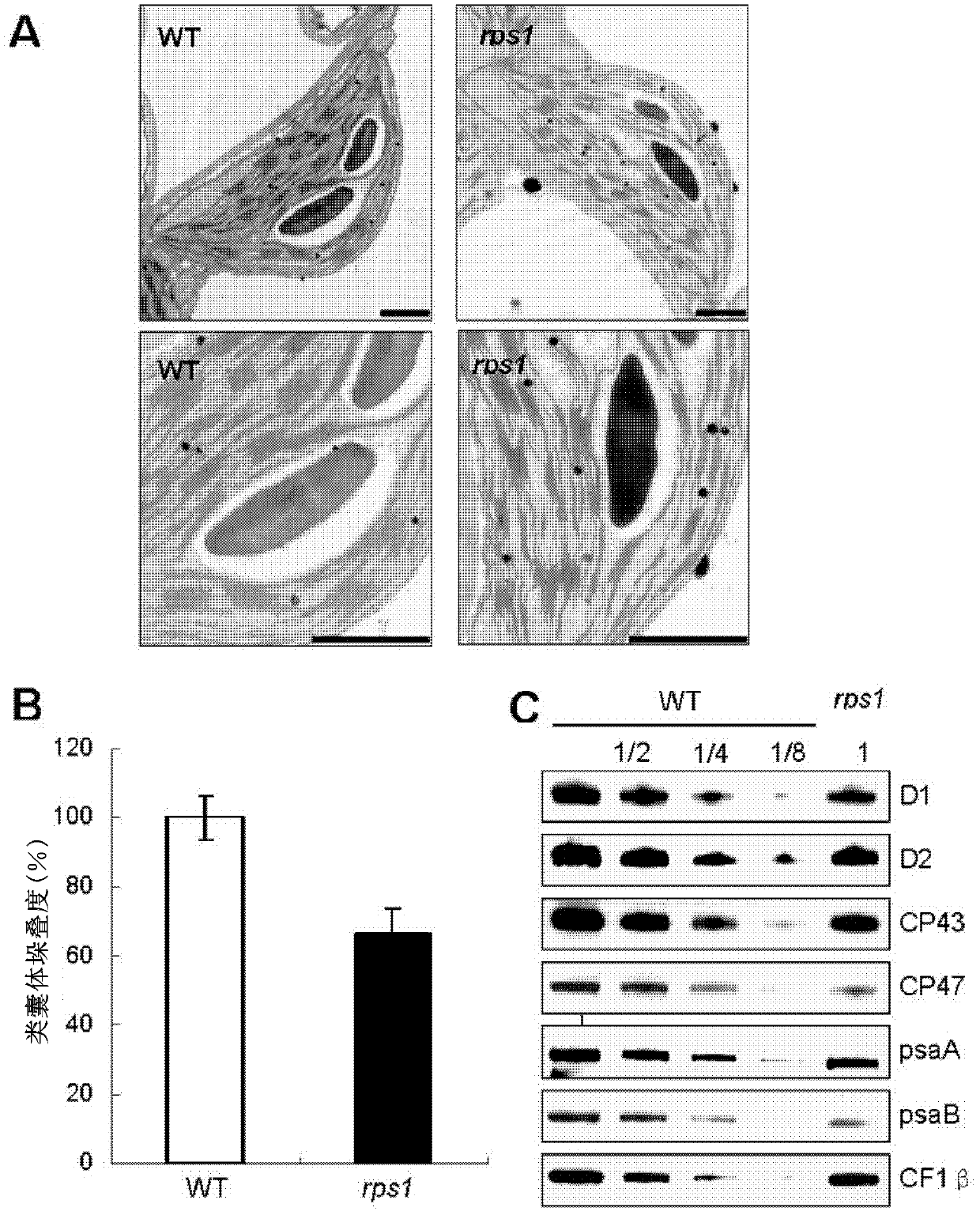
[0106] Downregulation of RPS1 expression reduces the stability of the thylakoid membrane system in a dose-dependent manner
[0107] 1. Thylakoid stacking degree determination method: reference (Khatoon et al., 2009), with appropriate modifications:
[0108] 1). Take 3-4g leaves of Arabidopsis thaliana, add 20ml ice-cold GB buffer solution, and quickly grind it in a pre-cooled mortar until it is homogenized;
[0109] 2). The homogenate is filtered with dust-free paper (2-4 layers);
[0110] 3). The filtrate was centrifuged at 4°C, 2600g, for 3min;
[0111] 4). Discard the supernatant, add 10ml RB buffer, and resuspend;
[0112] 5). Centrifuge at 2600g for 3min at 4°C;
[0113] 6). Repeat steps 4 and 5 once;
[0114] 7). Resuspend the precipitate in Solution C, measure the chlorophyll concentration with the acetone method, and adjust the chlorophyll concentration to 0.25mg / ml with Solution C;
[0115] 8). Incubate at 4°C for 15 minutes in the dark;
[0116] 9). Add digiton...
Embodiment 3
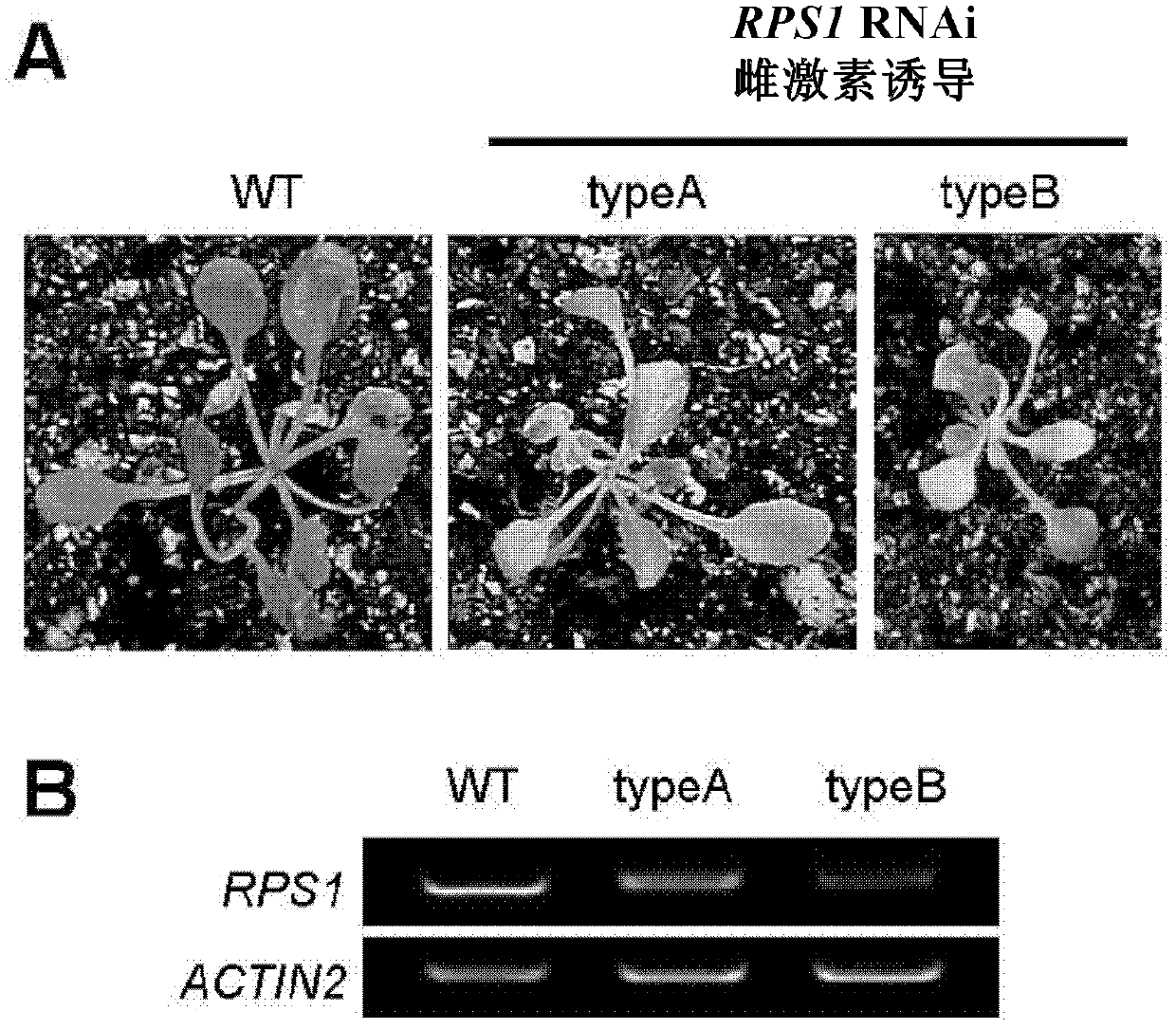
[0126] To construct an estrogen-inducible RPS1-RNAi binary vector, the construction method is as follows:
[0127] The sequence between 25 bp upstream and 457 bp downstream of the initiation codon ATG of RPS1 was amplified by RT-PCR.
[0128] The sense fragment primers are:
[0129] Upstream primer: 5′AAACCCGGGCTCGAGCTGTGTGAGTGAGTGAGACTC-3′
[0130] (SEQ ID NO.: 14)
[0131] Downstream primer: 5′-CGCTCTAGATGACAAACTCTTCCACCATAC-3′
[0132] (SEQ ID NO.: 15)
[0133] Antisense fragment primers are:
[0134] Upstream primer: 5′-AAAGAGCTCCTCGAGCTGTGTGAGTGAGTGAGACTC-3′
[0135] (SEQ ID NO.: 16)
[0136] Downstream primer: 5′-CACGCGGCCGCTGACAAACTCTTCCACCATAC-3′
[0137] (SEQ ID NO.: 17)
[0138] PCR conditions: annealing at 54°C for 15s, extension at 72°C for 0.5min, 30 cycles.
[0139] The amplified sense fragment and antisense fragment were connected to the commercially available pMD19-T vector by TA cloning method, and then transferred into the commercially available host...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com