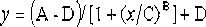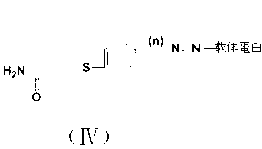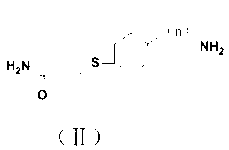Acrylamide hapten, artificial antigen, antibody and preparation method and application thereof
A technology of acrylamide and artificial antigen, which is applied in the field of food safety immunoassay, can solve the problems of poor repeatability and low titer of antibody preparation, and achieve the effects of short time, enhanced structural characteristics and high derivation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Preparation of hapten BI (n=1):
[0045] Take 0.212 g (2.9 mmoL) of acrylamide and dissolve it in 400 μL of carbonate buffer at pH 9.6, and take 0.1 g (0.5 mmoL) of p-mercaptophenylacetic acid and dissolve it in 800 μL of anhydrous methanol. The above-mentioned anhydrous methanol solution of p-mercaptophenylacetic acid was slowly added dropwise into the carbonate buffer of acrylamide, and stirred at 37 °C for 1 h in the dark. After the reaction was completed, use vacuum filtration method, add 2 mL of primary water to wash, take the precipitate, and dry it in vacuum at 37°C to obtain 256 mg of white solid 4-(3-amino-3-oxobutylthio)phenylacetic acid , and the yield was 82.05%. ESI-MS analysis (negative) m / z 239 [M-H]-; 1H NMR (CD3OD, 400 MHz) d 7.206-7.221 (d, J = 6.0 Hz, 2H), 6.670-6.685 (d, J = 6.0 Hz, 2H), 2.942-2.966 (m, 2H), 2.406-2.431 (m, 2H).
Embodiment 2
[0046] Example 2 Preparation method of hapten BII (n=0)
[0047] Take 0.284 g (3.99 mmoL) of acrylamide and dissolve it in 400 μL of 0.1 mol / L carbonate buffer solution with a pH of 9.6, and take 0.1 g (0.79 mmoL) of p-aminothiophenol and dissolve it in 800 μL of absolute ethanol. The above anhydrous methanol solution of p-aminothiophenol was slowly added dropwise into the acrylamide carbonate buffer, and stirred at 37 °C for 1 h in the dark. After the reaction, the mixed solution was adjusted to pH 3 with 4 M hydrochloric acid, extracted with ethyl acetate, the aqueous layer was adjusted to pH 9.6 with 4 M sodium carbonate, extracted with ethyl acetate, the mixed solution was poured into saturated sodium chloride, and brown-red Oily liquid 4-(3-amino-3-oxopropylthio)aniline 297 mg, yield 77.34%. ESI-MS analysis (negative) m / z 196 [M-H]-; 1 H NMR (CD 3 OD, 400 MHz) d 7.335-7.349 (d, J = 5.6 Hz, 2H), 7.233-7.247 (d, J = 5.6 Hz, 2H), 3.579 (s, 2H), 3.144-3.168 (m, 2H),...
Embodiment 3
[0048] Example 3 Immunogen / coating preparation
[0049] The difference between the preparation of the immunogen and the coating source lies in the carrier protein, the immunogen carrier protein uses keyhole limpet hemocyanin (KLH), and the coating source carrier protein uses ovalbumin (OVA). The preparation method of the immunogen is used as an example in the following.
[0050] Active ester method: Dissolve 0.0239 g (0.1 mmol) of hapten BⅠ (n=1) in 0.5 mL of dimethylformamide (DMF), stir and add 0.0512 g (0.2 mmol) of 1,3-dicyclohexylcarbodi Imine (DCC) and 0.023 g (0.2 mmoL) NHS were reacted overnight at 4 °C with magnetic stirring, and the supernatant was liquid A after centrifugation. Weigh 0.02 g of carrier protein KLH or OVA and dissolve it in 2 mL of PBS (pH8.0) solution with a concentration of 0.1 mol / L, stir and dissolve to prepare solution B. Under magnetic stirring, liquid A was gradually dropped into liquid B, and reacted at 4 °C for 12 h. After centrifugation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com