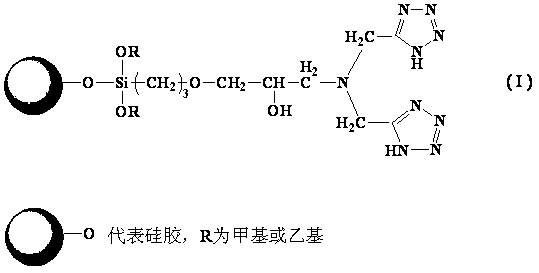
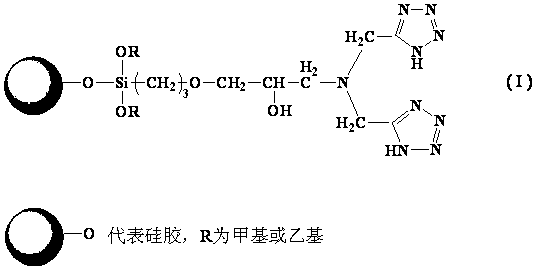
Fixed metal affinity chromatographic stationary phase based on strong chelating ligand and preparation method of stationary phase
A chromatographic stationary phase and affinity technology, applied in the field of chromatographic separation, can solve the problems of metal ion loss of target products, protein drug contamination, inactivation and other problems, and achieve the effect of good separation effect, long service life and reduction of loss.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of iminobis(methylenetetrazole) chromatographic stationary phase (method 1)
[0033] Preparation of bis-cyanosilane coupling agent: Weigh 1.5 g of iminodiacetonitrile (IDAN), dissolve it in 30 mL of methanol, add it to a 100 mL flask, add 1.2 mL of KH-560 dropwise, and react at 80°C for 48 h (the ratio of IDAN to KH-560 is 3:1). After the reaction was completed, methanol was evaporated to obtain a bis-cyanosilane coupling agent.
[0034] In a 100 mL flask, add bis-cyanosilane coupling agent and 50 mL of anhydrous toluene, heat to completely dissolve the bis-cyano silane coupling agent, then add 2 g of dry silica gel, put the flask into ultrasonic dispersion for 10 min, Then reflux at 110°C for 24 h. After the reaction was completed, it was washed several times with toluene, acetone, water and methanol successively. Vacuum drying at 50°C yielded iminodiacetonitrile-bonded silica gel. Elemental analysis results: carbon content 3.116%, hydrogen c...
Embodiment 2
[0037] Example 2: Preparation of iminobis(methylenetetrazole) chromatographic stationary phase (method 2)
[0038]Add 2 g of dry silica gel to a 100 mL three-necked flask, add 50 mL of anhydrous toluene, sonicate for 10 min, then slowly add 2 mL of KH-560 into the flask, and reflux at 110 °C for 20 h. After the reaction is completed, wash with toluene, water, and methanol several times in sequence, and dry under vacuum at 50° C. to obtain epoxy-based silica gel.
[0039] Weigh 1.5 g of IDAN, dissolve it in 30 mL of methanol, add it into a 100 mL flask, then add the above-mentioned epoxy-based silica gel, and react at 80 °C for 24 h while stirring. After the reaction was completed, it was washed several times with distilled water and methanol, and vacuum-dried at 50° C. to obtain iminodiacetonitrile-bonded silica gel.
[0040] Add iminodiacetonitrile-bonded silica gel into a 100 mL flask, then add 3.0 g sodium azide, 2.7 g ammonium chloride and 50 mL DMF, ultrasonically disper...
Embodiment 3
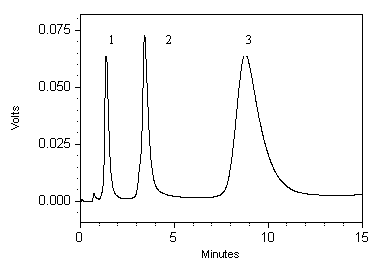
[0041] Example 3: The chromatographic stationary phase prepared in Example 1 was used to pack a chromatographic column (4.6 mm×100 mm) to chelate Zn 2+ , to separate a mixture of RNase-A, cytochrome-C and lysozyme ( figure 2 ). The separation effect is good, and the mass recovery rate of protein is greater than 95%. Taking lysozyme as an example, the activity recovery rate is greater than 93%. The column was continuously washed with 1.0 mL / min mobile phase (20 mmol / L NaAc-HAc, pH 5.0) for 2 days, Zn 2+ The loss of chelation amount is less than 8%, which is better than that of chelated Cu under the same conditions 2+ The iminodiacetic acid stationary phase metal ion loss is reduced by 56%.
[0042] Chromatographic column: 100×4.6 mm stainless steel column; mobile phase: A (equilibrium liquid), 20 mmol / L phosphate (pH 7.0), mobile phase B (eluent): A+1.0 mol / L NaCl (pH 7.0) ; Mobile phase flow rate: 1.0 mL / min; Linear gradient: 20 min, 100% A- 100% B, 100% B for 10 min. Sta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com