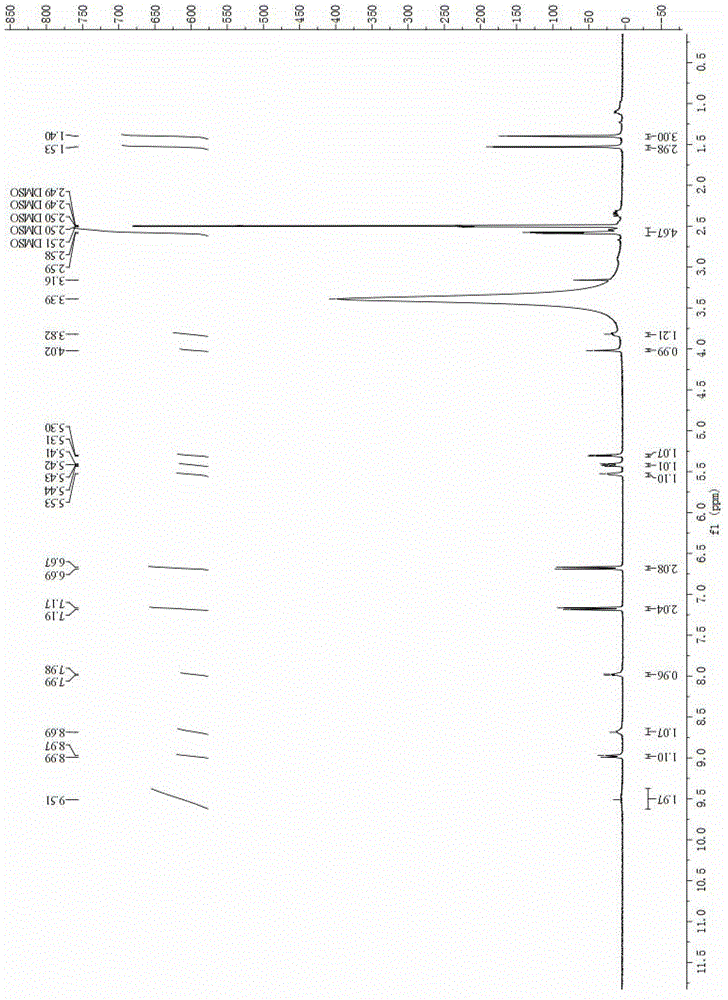
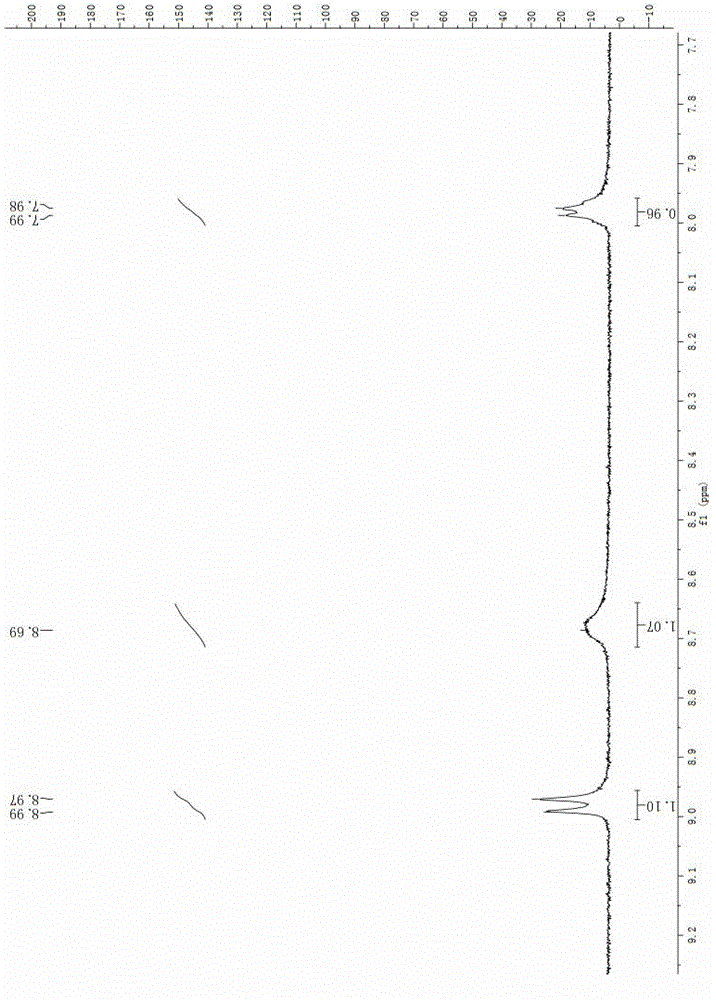
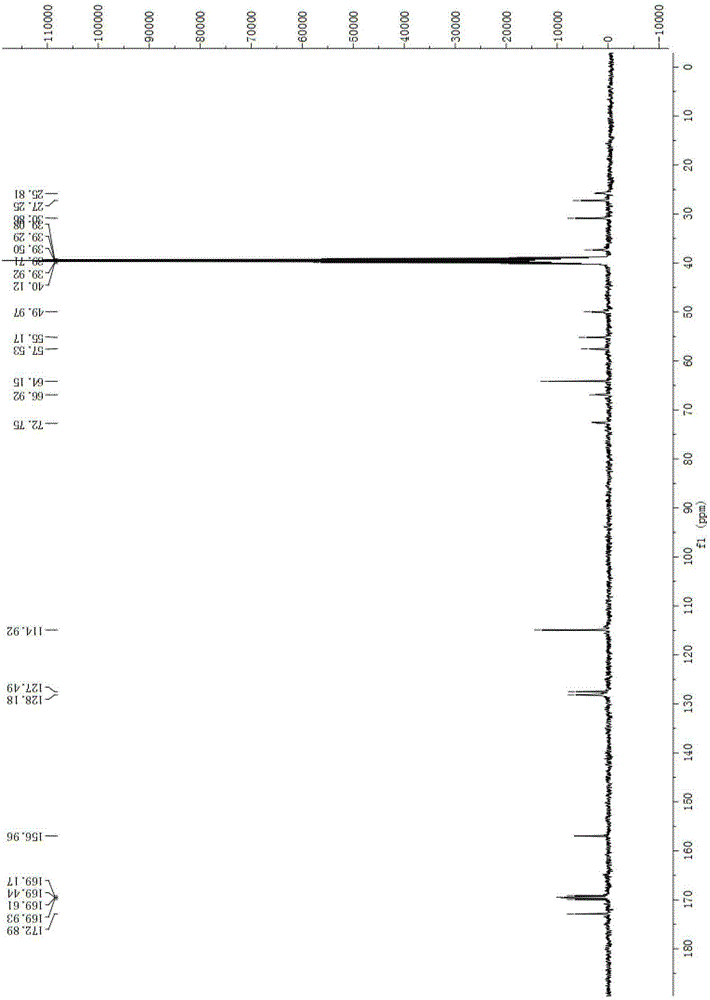
A kind of preparation method of apoxicillin trihydrate
A technology of apoxicillin trihydrate and mixed solvents, which is applied in the field of pharmaceutical production, can solve the problems of long synthetic route, high difficulty, and increased impurity spectrum, and achieve the effect of short synthetic route, large industrial value and improved quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0042] The preferred embodiments of the present invention will be described in detail below.
[0043] The present invention aims to protect the whole synthetic route, and the following synthetic examples are only to prove that the synthetic route is feasible. But the content of invention protection is not limited to examples.
[0044] 1) Preparation of aspartic acid-β-methyl ester hydrochloride
[0045] Weigh 133.0g (1.0mol) of D-aspartic acid into a 2L three-neck flask, add 300mL of methanol and stir at 25-35°C (the solid does not dissolve and becomes white and turbid), and another 109.0g (1.1mol, 1.1 eq), use methanol 300mL, stir to dissolve, add BTC methanol solution dropwise to aspartic acid reaction liquid. Control the rate of addition so that the temperature of the reaction solution is not higher than 40°C. After the dropwise addition was completed, the reaction was stirred at 25-35° C. for 30-60 min, and the reaction was detected by TLC. (take a sample of 0.5mL, add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com