Secretory expression method of bacteriocin lacticin Q
An expression method and an expression vector technology, which are applied in the field of secretion and expression of bacteriocin lacticin Q, can solve problems such as unfavorable large-scale production and application, and difficulty in separation and purification of wild bacterial bacteriocin, and achieve the goal of simplifying the separation and purification process and increasing protein production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Construction of embodiment 1 SUMO-lacticin Q fusion gene
[0042] According to the amino acid sequence of lacticin Q, its gene sequence (as shown in 345-504 in the sequence table SEQ ID NO.1) was synthesized, loaded into T vector, and transformed into Escherichia coli DH5α. The primers P1 (as shown in SEQ ID NO.3 in the sequence table) and P2 (as shown in SEQ ID NO.4 in the sequence table) were designed using the software Premier5.0 to amplify the lacticin Q gene.
[0043] PCR reaction system:
[0044]
[0045] Reaction program: denaturation at 95°C for 5min; denaturation at 95°C for 30Sec; annealing at 55°C for 30Sec; extension at 72°C for 1min, 25 cycles, and finally extension at 72°C for 8min. After cutting the target band by 2% agarose gel electrophoresis, it was purified and recovered with a DNA gel extraction kit.
[0046] Since the PCR product amplified by Taq DNA polymerase contains prominent A bases at both ends, and the carrier pET SUMO has been linearize...
Embodiment 2
[0047] Example 2 Expression plasmid construction
[0048] 1) PCR amplification of SUMO-lacticin Q fusion gene
[0049] According to the SUMO-lacticin Q fusion gene sequence and the pWB980 cloning site, primers P3 (as shown in the sequence table SEQ ID NO.5) and P4 (as shown in the sequence table SEQ ID NO.6) were amplified from the plasmid pET SUMO-lacticin Q. Add the coding sequence of the SUMO-lacticin Q fusion protein gene containing the His tag (as shown in the sequence table SEQ ID NO.1), wherein primer P3 is introduced into the HindIII site, and primer P4 is introduced into the XbaI site, so that the fusion gene is cloned into the pWB980 plasmid .
[0050] PCR reaction system:
[0051]
[0052] Reaction program: denaturation at 95°C for 5min; denaturation at 95°C for 30Sec; annealing at 49.5°C for 30Sec; extension at 72°C for 1min, 20 cycles, and finally extension at 72°C for 8min. After cutting the target band by 2% agarose gel electrophoresis, it was purified and...
Embodiment 3
[0055] The acquisition of embodiment 3 recombinant lacticin Q
[0056] 1) secreted expression
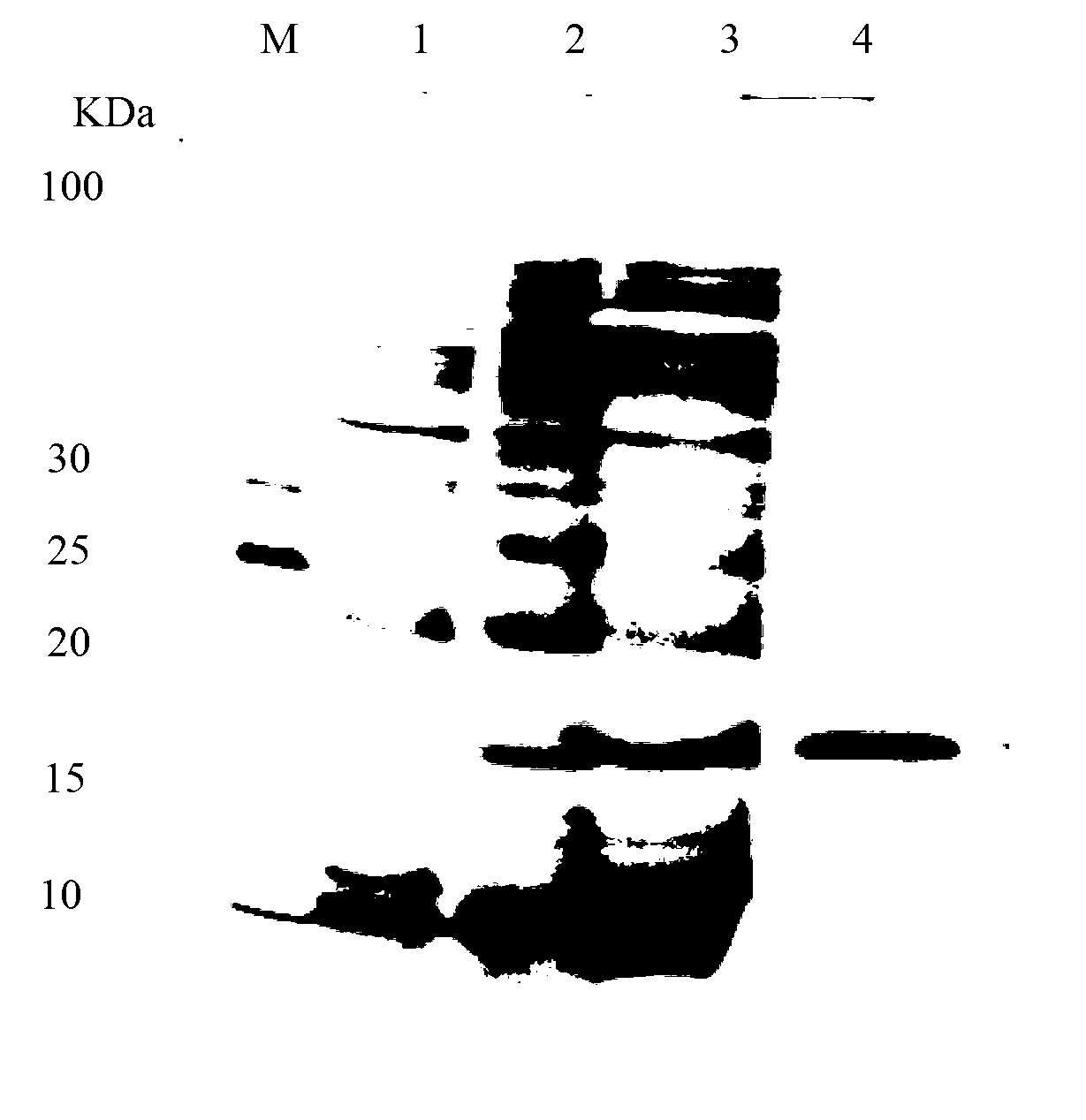
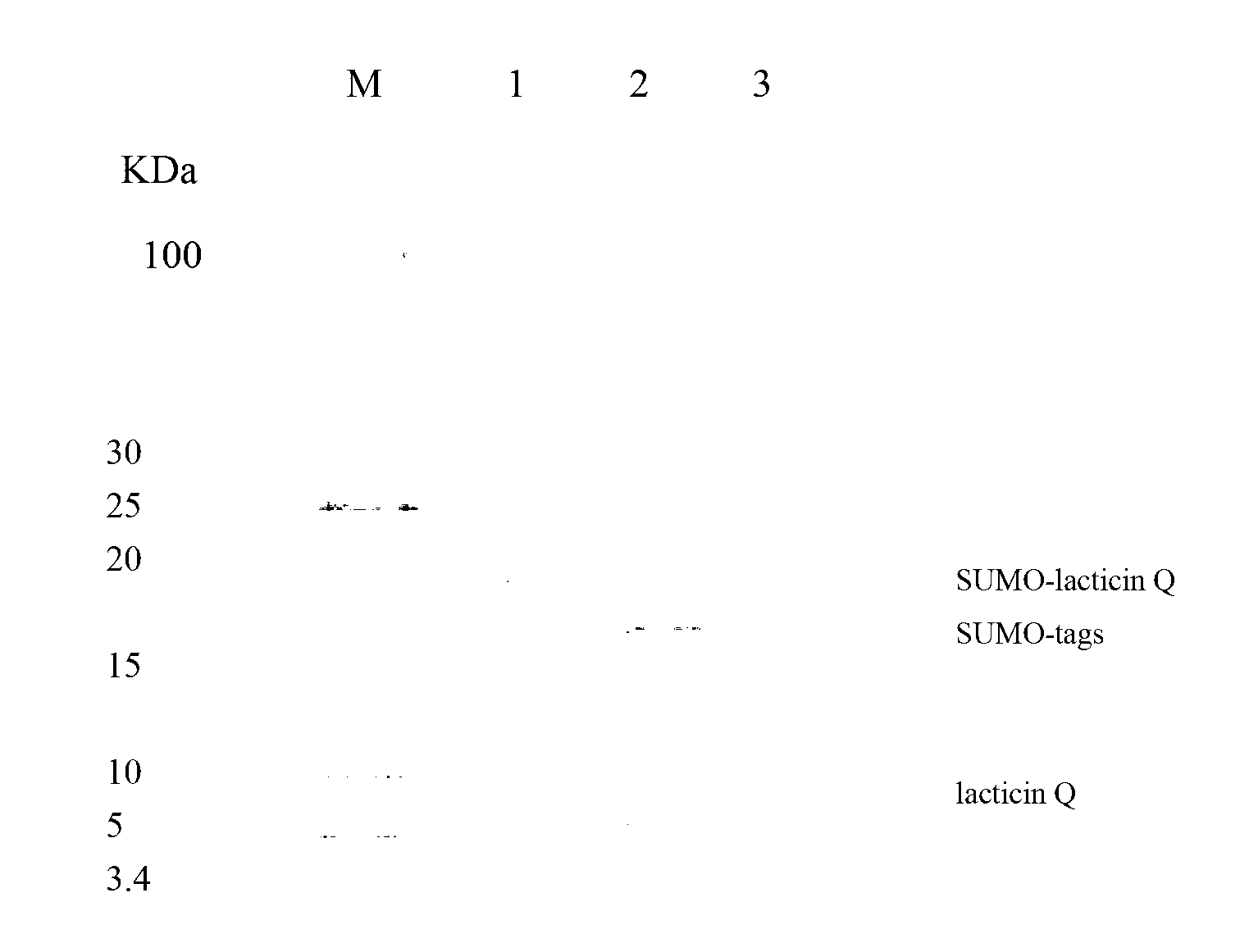
[0057] A single colony of the screened positive recombinant bacteria WB600 / pWB980 / SUMO-lacticin Q was inoculated into LB medium, and shaken at 150 rpm for 12 hours at 37°C. Then it was transferred to fresh LB medium at a ratio of 4%, and was shaken at 37°C and 200rpm for 36h to secrete and express SUMO-lacticin Q in the medium, and the expression effect was analyzed by Tricine-SDS-PAGE ( figure 1 ), SUMO-lacticin Q accounted for more than 21% of the total supernatant protein.
[0058] Tricine-SDS-PAGE analysis
[0059] The configuration of stacking gel and separating gel is as follows
[0060]
[0061] After the gel is solidified, add a quarter volume of 5xloading buffer to the sample, boil for 5min, and load the sample for electrophoresis after cooling.
[0062] The voltage of the stacking gel is 60V, and the voltage of the separating gel is 120V.
[0063] After electrophor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com