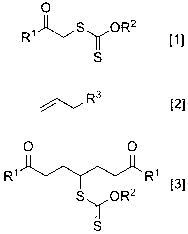
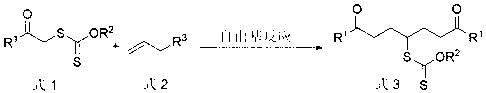Preparation method of symmetric 1,7-dicarbonyl compound
A dicarbonyl compound, symmetrical technology, applied in the direction of organic chemistry, can solve the problems of limited scope of application, poor tolerance of groups, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Dithiocarbonic acid O -Ethyl- S -(1,7-Diphenyl-1,7-dioxohept-4-yl)ester 3a preparation of
[0064] Dithiocarbonic acid O -Ethyl- S- Dissolve (2-phenyl-2-oxoethyl) ester (2.2 mmol) and methallyl sulfone (1 mmol) in 1.5 mL of 1,2-dichloroethane, vacuumize the system, and fill Nitrogen was added, the oil bath was heated to reflux, lauroyl peroxide (DLP) (5 mol % xanthate, 46 mg) was added, and then DLP (10 mol %, 92 mg) was added every 60 min until 45 mol% DLP was added . Continue the reflux reaction for 10 hrs, spin off the solvent, and the resulting crude product is separated by silica gel column chromatography (eluent petroleum ether: ethyl acetate = 15:1 to 8:1) to obtain dithiocarbonic acid O -Ethyl- S -(1,7-diphenyl-1,7-dioxohept-4-yl) ester, light yellow solid 325 mg, yield 81%, melting point 72-73 o c. 1 H NMR (CDCl 3 , 400 MHz) ( δ , ppm) 1.36 (t, J = 7.1 Hz, 3H, CH 3 ), 2.09 (dddd, J = 14.8, 8.9, 7.2, 6.5 Hz, 2H in 2CH 2 ), 2.31 (dddd, J = 14....
Embodiment 2
[0066] Dithiocarbonic acidO -Ethyl- S -[1,7-bis(4-methoxyphenyl)-1,7-dioxohept-4-yl]ester 3b preparation of
[0067] According to the method described in Example 1, with dithiocarbonic acid O -Ethyl- S -(2-p-methoxyphenyl-2-oxoethyl) ester and methallyl sulfone as raw materials to obtain dithiocarbonic acid O -Ethyl- S -[1,7-bis(4-methoxyphenyl)-1,7-dioxohept-4-yl]ester, white solid 360 mg, yield 78%, melting point 60-61 o c. 1 H NMR (CDCl 3 , 400 MHz) ( δ , ppm) 1.36 (t, J = 7.1 Hz, 3H, CH 3 ), 2.08 (dddd, J = 14.5, 8.5, 6.3, 6.0 Hz, 2H in 2CH 2 ), 2.27 (dddd, J = 14.5, 8.5, 6.7, 5.0 Hz, 2H in 2CH 2 ), 3.06~3.20 (m, 4H, 2CH 2 ), 3.86 (s, 6H, 2CH 3 ), 3.93 (tt, J = 8.5, 5.0 Hz, 1H, CH), 4.57 (q, J = 7.1 Hz, 2H, CH 2 ), 6.92 (d, J = 8.8 Hz, 4H, ArH), 7.94 (d, J = 8.8 Hz, 4H, ArH). 13 C NMR (CDCl 3 , 100 MHz) ( δ , ppm) 13.6, 29.0, 35.3, 50.8, 55.4, 69.9, 113.6, 129.8, 130.2, 163.4, 197.6, 213.9.
Embodiment 3
[0069] Dithiocarbonic acid O -Ethyl- S -(2,8-dioxononan-5-yl)ester 3c preparation of
[0070] According to the method described in Example 1, with dithiocarbonic acid O -Ethyl- S -(2-oxopropyl) ester and methallyl sulfone as raw materials to obtain dithiocarbonic acid O -Ethyl- S -(2,8-dioxononan-5-yl) ester, pale yellow liquid 199 mg, yield 72%. 1 H NMR (CDCl 3 , 400 MHz) ( δ , ppm) 1.43 (t, J = 7.1 Hz, 3H, CH 3 ), 1.83 (dddd, J = 14.7, 8.5, 7.7, 6.7 Hz, 2H in 2CH 2 ), 2.04 (dddd, J = 14.7, 8.5, 7.5, 5.0 Hz, 2H in 2CH 2 ), 2.14 (s, 6H, 2CH 3 ), 2.59 (ddd, J = 15.0, 7.7, 6.7 Hz, 2H in 2CH 2 ), 2.63 (dt, J = 15.0, 7.5 Hz, 2H in 2CH 2 ), 3.71 (tt, J = 8.5, 5.0 Hz, 1H, CH), 4.64 (q, J = 7.1 Hz, 2H, CH 2 ). 13 C NMR (CDCl 3 , 100 MHz) ( δ , ppm) 13.7, 28.2, 30.0, 40.5, 50.5, 70.1, 207.6, 214.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com