protein-fes 2 Bioconjugated nanofibers and their preparation and use
A technology of nanofiber, -fes2, applied in the field of nano
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
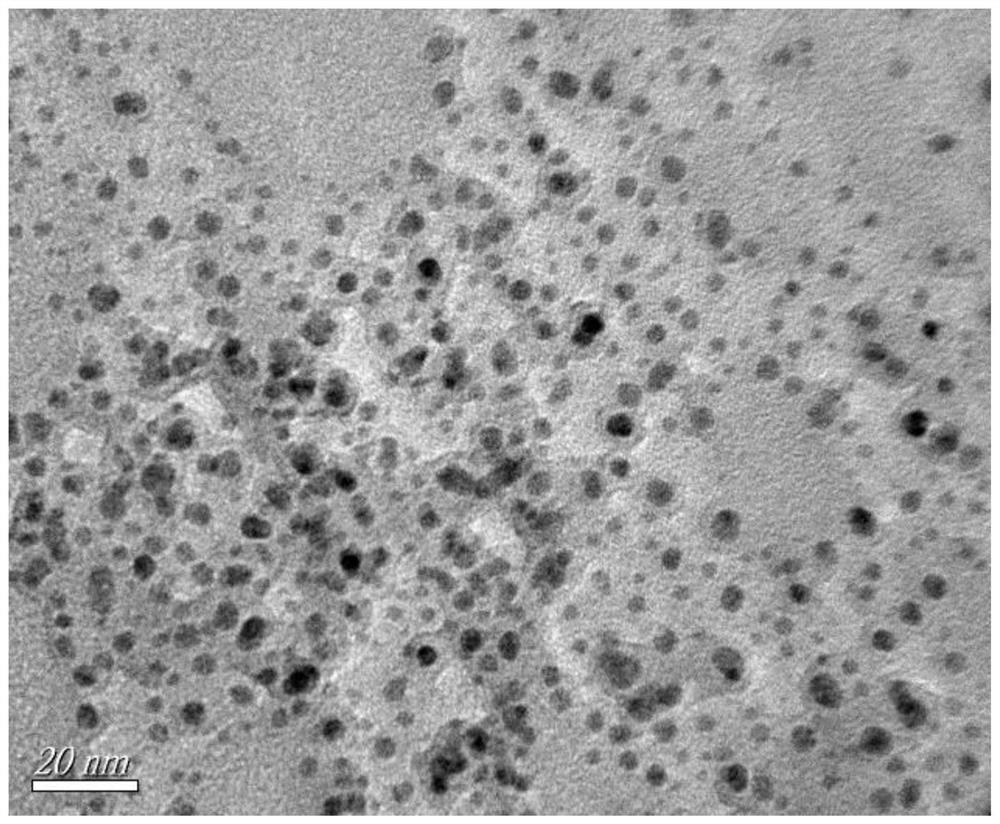
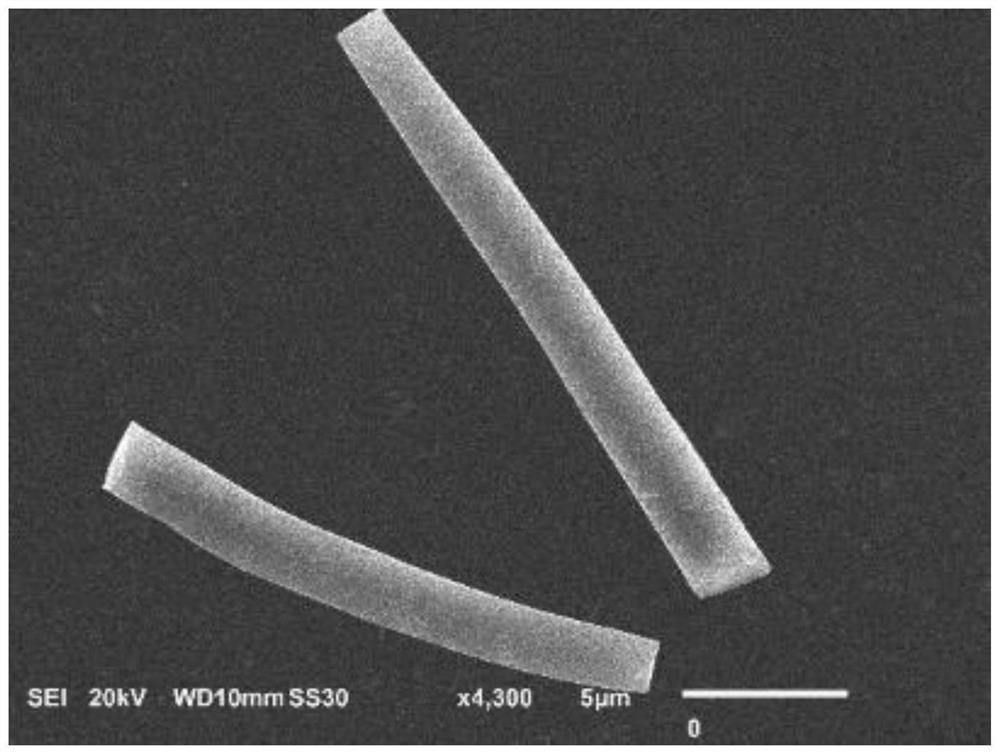
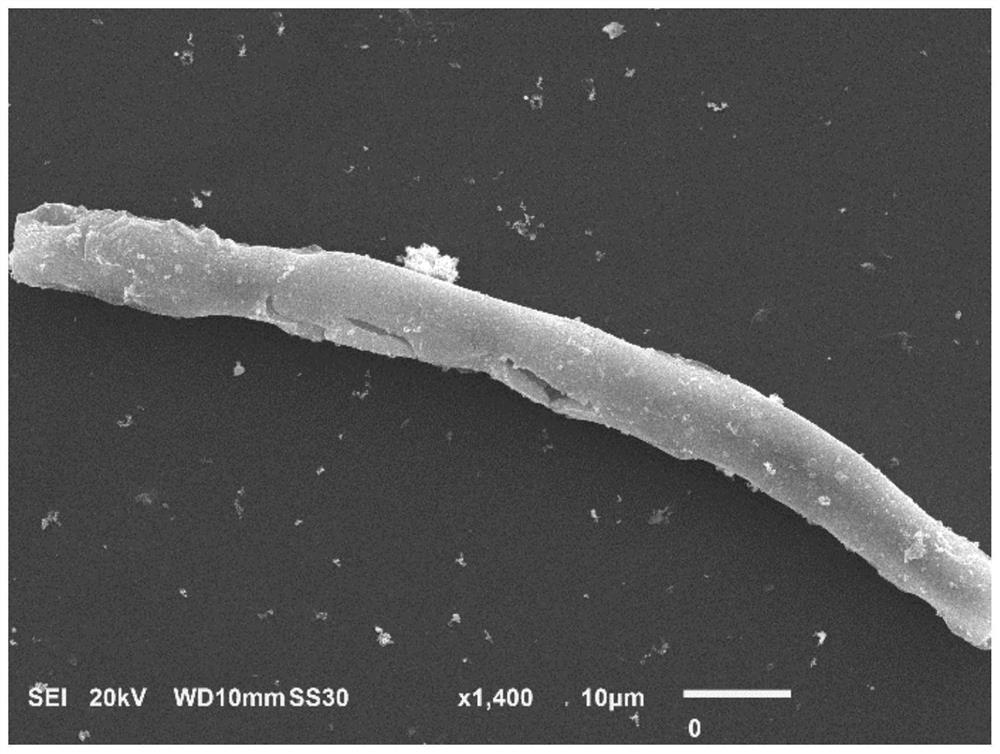
[0022] Embodiment 1 (FeS 2 @BDA@Au optimal preparation scheme): In 9mL aqueous solution, add 2mmol bovine serum albumin and 2mmol dopamine (molar ratio of bovine serum albumin to dopamine 1:1), and stir magnetically for the first time at room temperature for 30min to make the solution evenly mixed. At room temperature, add ferrous ammonium sulfate solution to the above protein solution (volume of ferrous ammonium sulfate solution is 1mL, molar ratio of bovine serum albumin to ferrous ammonium sulfate is 2:3), and after magnetic stirring for the second time for 30min, adjust After the pH value of the mixed solution was 9, the sodium sulfide solution was added to the mixed solution of the protein and ammonium ferrous sulfate (the volume of the sodium sulfide solution was 1 mL, and the mol ratio of sodium sulfide and ammonium ferrous sulfate was 4:1), and the third magnetic After stirring for 30min, transfer to a water bath at 25°C and stir magnetically for 6h to obtain FeS 2 @B...
Embodiment 2
[0023]Example 2: In 9mL aqueous solution, add 2mmol bovine serum albumin and 1mmol dopamine (the molar ratio of bovine serum albumin to dopamine is 2:1), and stir magnetically for the first time at room temperature for 30min to make the solution evenly mixed. At room temperature, add ferrous ammonium sulfate solution to the protein solution (the volume of ferrous ammonium sulfate solution is 1 mL, the molar ratio of bovine serum albumin to ferrous ammonium sulfate is 2:3), and after magnetic stirring for the second time for 30 min, After the pH value of the mixed solution was adjusted to 9, the sodium sulfide solution was added to the mixed solution of the protein and ammonium ferrous sulfate (the volume of the sodium sulfide solution was 1 mL, and the mol ratio of sodium sulfide to ammonium ferrous sulfate was 4:1), the third time After magnetic stirring for 30 minutes, transfer to a water bath at 25°C and magnetically stir for 6 hours to obtain FeS 2 @BDA nanofibers. Will F...
Embodiment 3
[0024] Example 3: In 9 mL of aqueous solution, 2 mmol of bovine serum albumin and 2 mmol of dopamine were added (the molar ratio of bovine serum albumin to dopamine was 1:1), and the solution was mixed uniformly by magnetic stirring for the first time at room temperature for 30 min. At room temperature, add ferrous ammonium sulfate solution to the protein solution (the volume of ferrous ammonium sulfate solution is 1 mL, the molar ratio of bovine serum albumin to ferrous ammonium sulfate is 2:1), and after magnetic stirring for the second time for 30 min, After the pH value of the mixed solution was adjusted to 9, the sodium sulfide solution was added to the mixed solution of the protein and ammonium ferrous sulfate (the volume of the sodium sulfide solution was 1 mL, and the mol ratio of sodium sulfide to ammonium ferrous sulfate was 4:1), the third time After magnetic stirring for 30 minutes, transfer to a water bath at 25°C and magnetically stir for 6 hours to obtain FeS 2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| photothermal conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com