Sulfone electrolyte for lithium-air battery
A technology of sulfone electrolyte and lithium-air battery, which is applied in the direction of fuel cell half-cell and secondary battery-type half-cell, etc. It can solve the problems of irreversible decomposition of positive electrode, low reversible efficiency, unfavorable battery cycle, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In a glove box filled with high-purity argon, weigh 9 g of dimethyl sulfoxide and 1 g of LiN(SO 2 CF 3 ) 2 , made into a mixed solution, stirred evenly and left to stand for 2 hours to obtain a sulfone electrolyte.
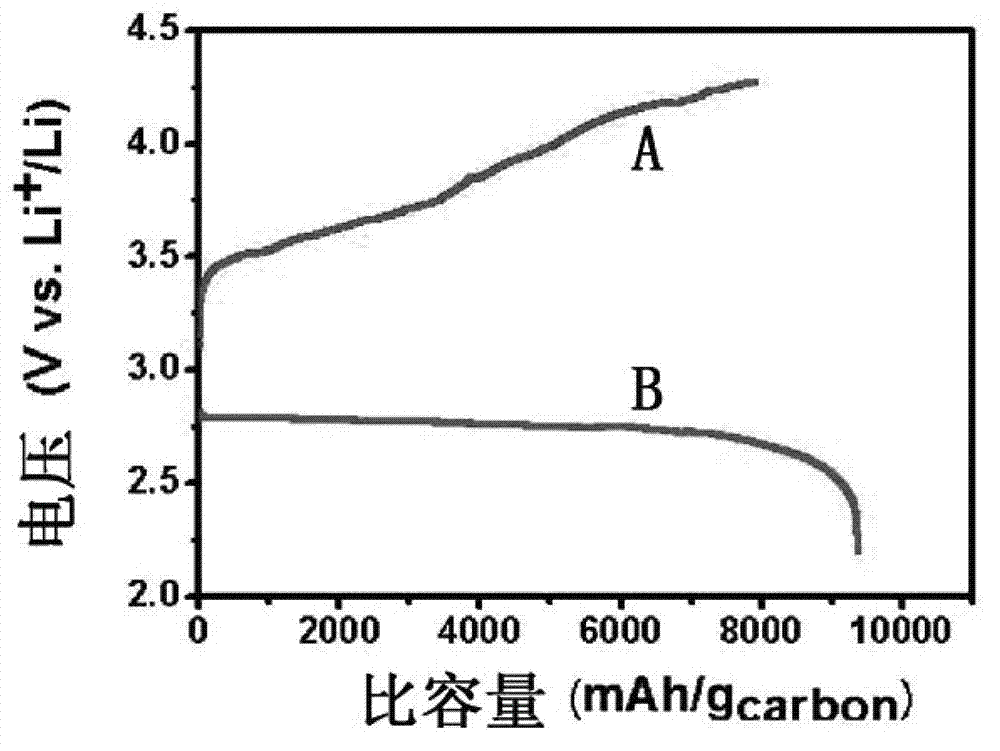
[0024] Using KB carbon as the positive electrode, metal lithium sheet as the negative electrode, and the sulfone electrolyte prepared above as the electrolyte, a CR2025 button battery was assembled in a glove box filled with argon. The above coin cell was charged at room temperature at 0.05mAcm -2 The constant current charge and discharge test is carried out in a pure oxygen atmosphere, and the charge and discharge cut-off voltage is 2-4.5V. figure 1 It is the first charge and discharge curve diagram of the lithium-air battery obtained in Example 1 of the present invention, figure 1 A is the discharge curve, and B is the charge curve. It can be seen from the figure that at 0.05mAcm -2 Under the current density, the first discharge specific capacity is ...
Embodiment 2
[0026] In a glove box filled with high-purity argon, weigh 9 g of sulfolane and 1 g of LiN(SO 2 CF 3 ) 2 , made into a mixed solution, stirred evenly and left to stand for 2 hours to obtain a sulfone electrolyte.
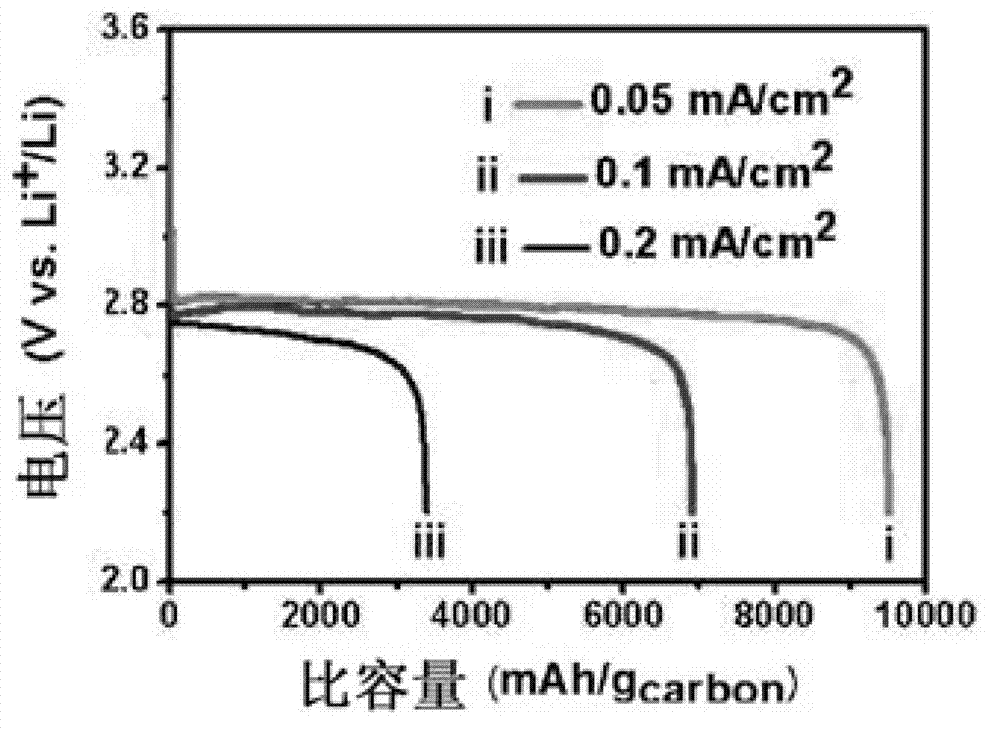
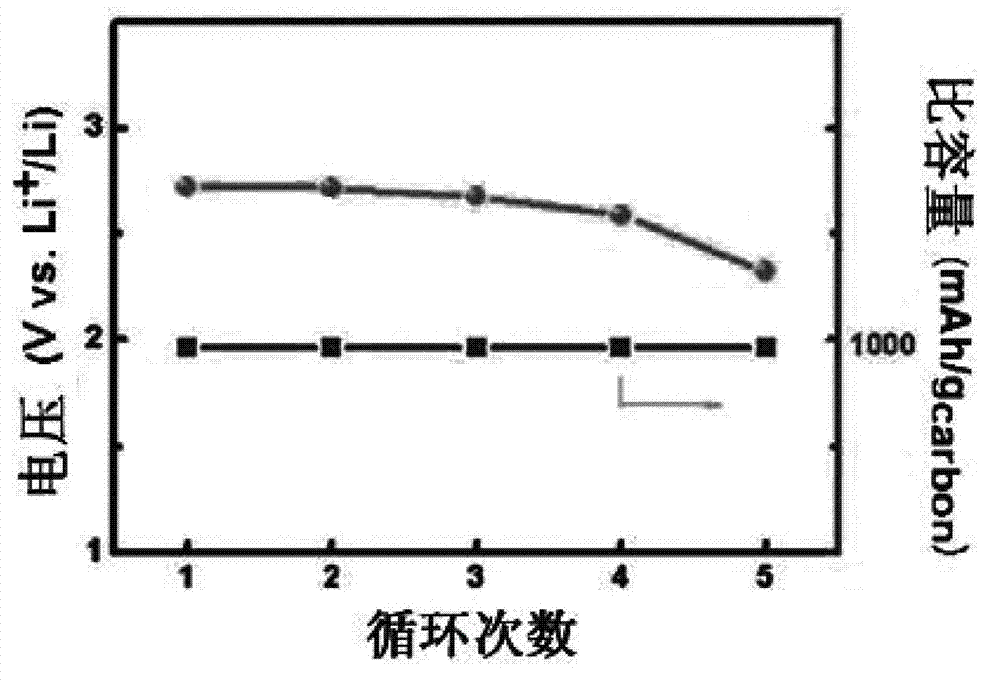
[0027] Using KB carbon as the positive electrode, metal lithium sheet as the negative electrode, and the sulfone electrolyte prepared above as the electrolyte, a CR2025 button battery was assembled in a glove box filled with argon. The above coin cell was charged at 0.1mAcm at room temperature -2 The constant current charge and discharge test is carried out in a pure oxygen atmosphere, and the charge and discharge cut-off voltage is 2-4.5V. image 3 The lithium-air battery that obtains for the embodiment 2 of the present invention is at 0.1mAcm -2 The cycle performance graph under the current density, it can be seen from the graph that at 0.1mAcm -2 The battery can be cycled stably for 10 times under the current density. Figure 4 The lithium-air battery rate ...
Embodiment 3
[0029] In a glove box filled with high-purity argon, weigh 8.5g sulfolane and 1gLiPF with a microanalytical electronic balance 6 , made into a mixed solution, weighed 0.5 g of additive propane sultone and added to the above solution, stirred evenly and left to stand for 2 hours to obtain a sulfone electrolyte.
[0030] Using KB carbon as the positive electrode, metal lithium sheet as the negative electrode, and the sulfone electrolyte prepared above as the electrolyte, a CR2025 button battery was assembled in a glove box filled with argon. The above coin cell was charged at room temperature at 0.05mAcm -2 The constant current charge and discharge test is carried out in a pure oxygen atmosphere, the charge and discharge cut-off voltage is 2-4.5V, the discharge platform is 2.7V, and the discharge capacity is 9200mAh / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com