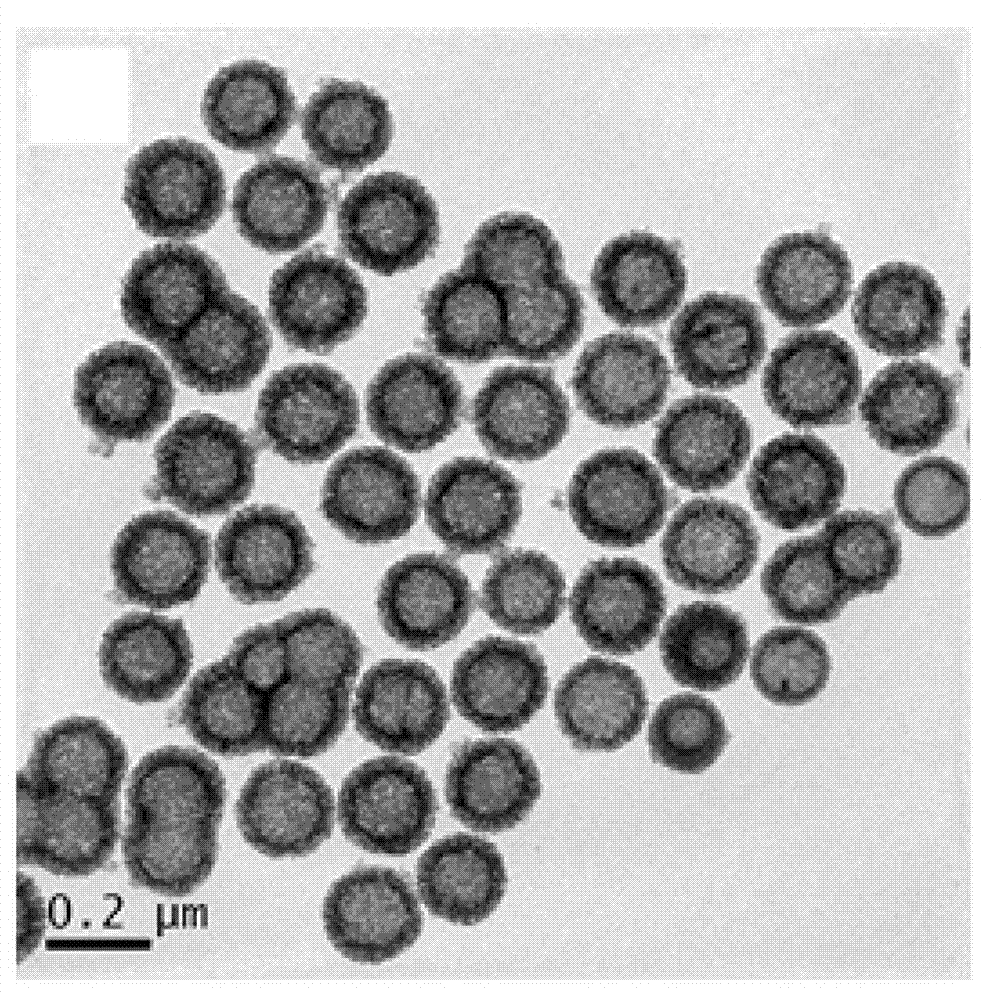
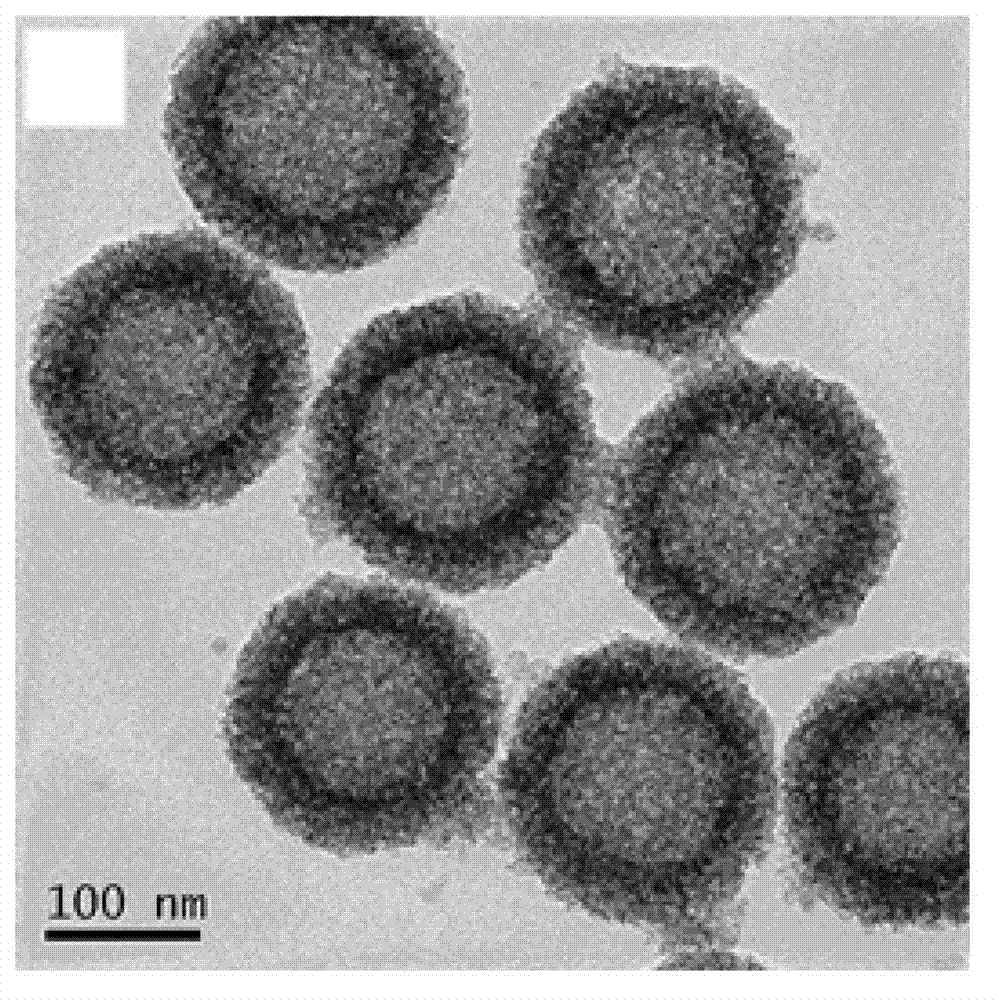
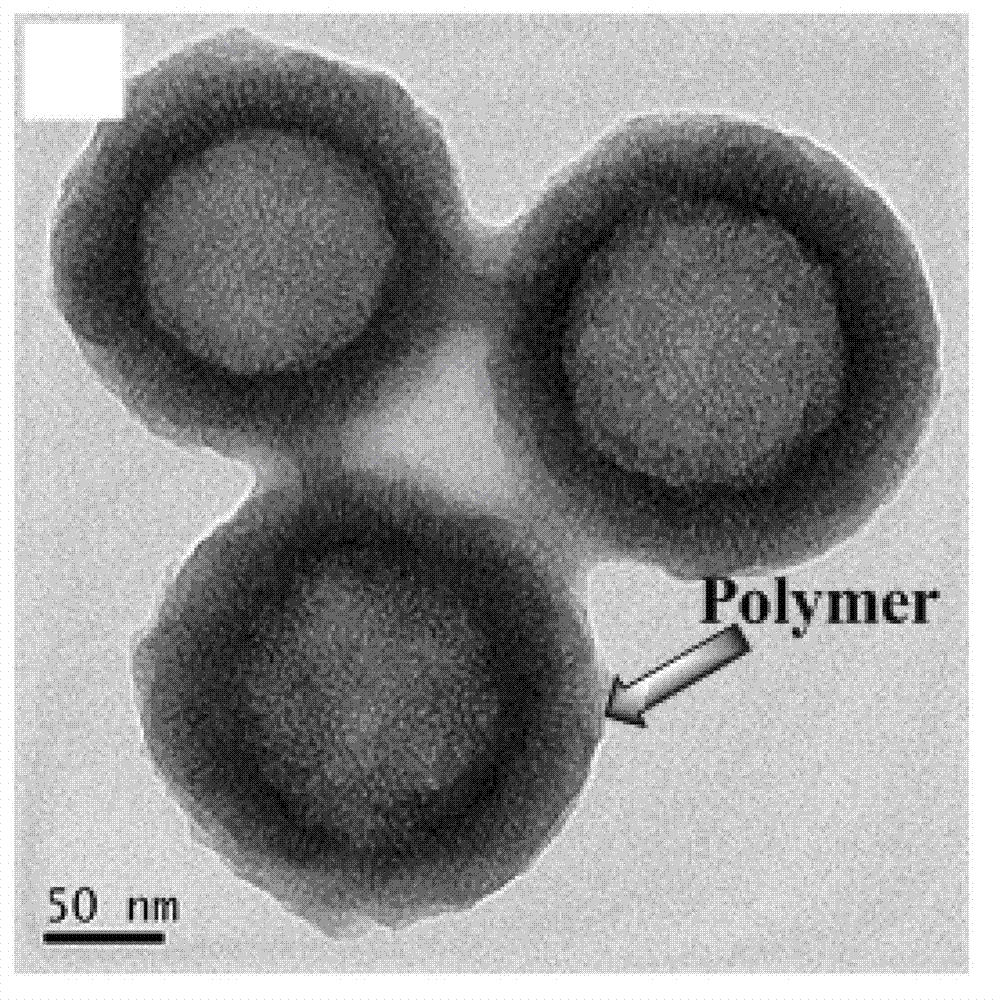
Amphiphilic polymer with tumor targeting property and visible light degradability and medicament carrier as well as preparation method thereof
An amphiphilic polymer and tumor-targeting technology, applied in the field of polymers, can solve problems such as application limitations, damage to normal cells or tissues, and unsatisfactory ultraviolet light penetration, and achieve controllable release and strong target effect on enrichment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The invention provides a method for preparing an amphiphilic polymer with tumor targeting and visible light degradability, comprising the following steps:
[0056] reacting anthraquinone, 3-iodo-1-propanol and sodium dithionite in a solvent to obtain the first compound;
[0057] React the first compound with the long-chain alkyl acid chloride in an organic solvent, and then continue to react the obtained reaction product with methacryloyl chloride to obtain a hydrophobic compound, the long-chain alkane in the long-chain alkyl acid chloride The number of carbon atoms in the group is greater than 8;
[0058] The hydrophobic compound, 4-cyanovaleric acid dithiobenzoic acid, N-(3-aminopropyl) methacrylate and hydroxyethyl methacrylate were subjected to reversible addition-fragmentation chain transfer in an organic solvent Polymerization reaction to obtain amphiphilic polymer;
[0059] reacting the amphiphilic polymer with thiocyanate-modified folic acid to obtain an amphi...
Embodiment 1
[0124] Synthesis of 4-cyanovaleric acid dithiobenzoic acid: under nitrogen protection and light-shielding conditions, 5 mL of potassium ferricyanide solution with a molar concentration of 0.05 mol / L was added dropwise to 8 mL with a molar concentration of 0.1 mol / L sodium dithiobenzoate solution to react, after reacting overnight, the reaction product obtained is filtered; the crude product obtained by filtration is recrystallized in ethanol to obtain bis-dithiobenzoyl;
[0125] Add the obtained bisdithiobenzoyl and 40 mL of ethyl acetate into a round-bottomed flask, then add 7 mmol of 4,4'-azobis(4-cyanovaleric acid) to it, and heat under nitrogen protection After reflux for 16 hours, the obtained crude product was separated by chromatographic column (ethyl acetate / n-hexane, 2 / 3) to obtain the product.
[0126] The product that the present invention will obtain carries out nuclear magnetic resonance spectrum (400MHz, CDCl 3 ) detection, the result is as follows: d(ppm):7.91(...
Embodiment 2
[0136] Add 30 mg of thiocyanate-modified folic acid into pyridine dissolved with 100 mg of the amphiphilic polymer with tumor targeting and visible light degradability prepared in Example 1, and stir at room temperature for 12 hours to obtain the product in the The amphiphilic polymer with tumor targeting and visible light degradability was obtained after precipitation in diethyl ether and vacuum drying.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com