Venlafaxine hydrochloride spansule and preparation method thereof
A technology of venlafaxine hydrochloride and sustained-release capsules, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., can solve the problems of increasing coating thickness and high cost, and achieve cost reduction. Low cost, stable quality, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Prescription with pill core:
[0030]
[0031] The preparation method is as follows:
[0032] Mix microcrystalline cellulose, hydroxypropyl methylcellulose and venlafaxine hydrochloride evenly. Use a soft material made of 5% polyvinylpyrrolidone solution to extrude in an extruder (screen diameter 0.8mm, extrusion speed: 25rpm), and put the extruded material in a spheronizer for spheronization (rotation speed 600-1000rpm, spheronization time 500 seconds ). Dry the material in a blast drying oven at 40°C-60°C for 2-4 hours, sieve, and sieve out the pellet cores larger than 16 mesh and less than 30 mesh.
[0033] Coating prescription:
[0034]
[0035]
[0036] Preparation method of coating solution: add hydroxypropyl methylcellulose into water, add talcum powder under the action of a shearing machine, add this solution into Eudragit NE30D, and stir.
[0037] Coating operation: take 500g of pill cores containing the drug and coat them with a fluidized bed bott...
Embodiment 2
[0042] Prescription with pill core:
[0043]
[0044] Preparation method: Mix microcrystalline cellulose and venlafaxine hydrochloride evenly. 3% hydroxypropyl methylcellulose solution was used as binder, and pellets were prepared by centrifugal granulation. Dry the material in a blast drying oven at 40°C-60°C for 2-4 hours, sieve, and sieve out the pellet cores larger than 16 mesh and less than 30 mesh.
[0045] Coating prescription:
[0046]
[0047]
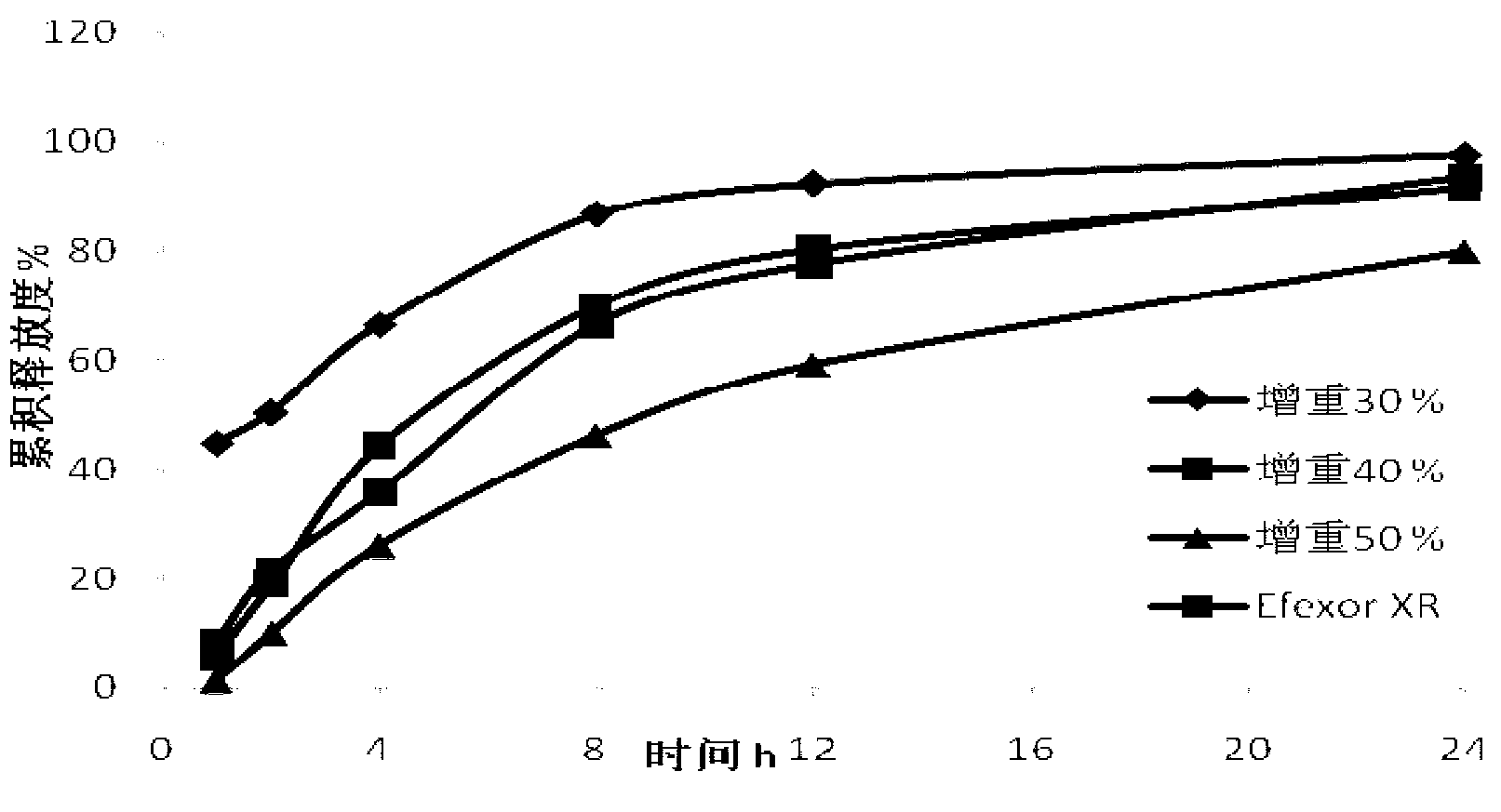
[0048] Coating operation: take 500 g of the 30-16 mesh-containing pill core prepared in Example 2, and coat the isolation layer and the controlled-release layer respectively by the method of bottom spraying to prepare sustained-release pellets. Coating weight gain (calculated by polymer weight) 30%, 40%.
[0049] Release degree inspection: According to the Chinese drug import registration standard X20000237, the release degree of the product was inspected, and compared with Efexor XR, a product of Wyeth Pharmaceuti...
Embodiment 3
[0053] Prescription with pill core:
[0054]
[0055] Preparation method: Mix microcrystalline cellulose and venlafaxine hydrochloride evenly. 3% hydroxypropyl methylcellulose solution was used as binder, and pellets were prepared by centrifugal granulation. Dry the material in a blast drying oven at 40°C-60°C for 2-4 hours, sieve, and sieve out the pellet cores larger than 16 mesh and less than 30 mesh.
[0056] Coating prescription:
[0057]
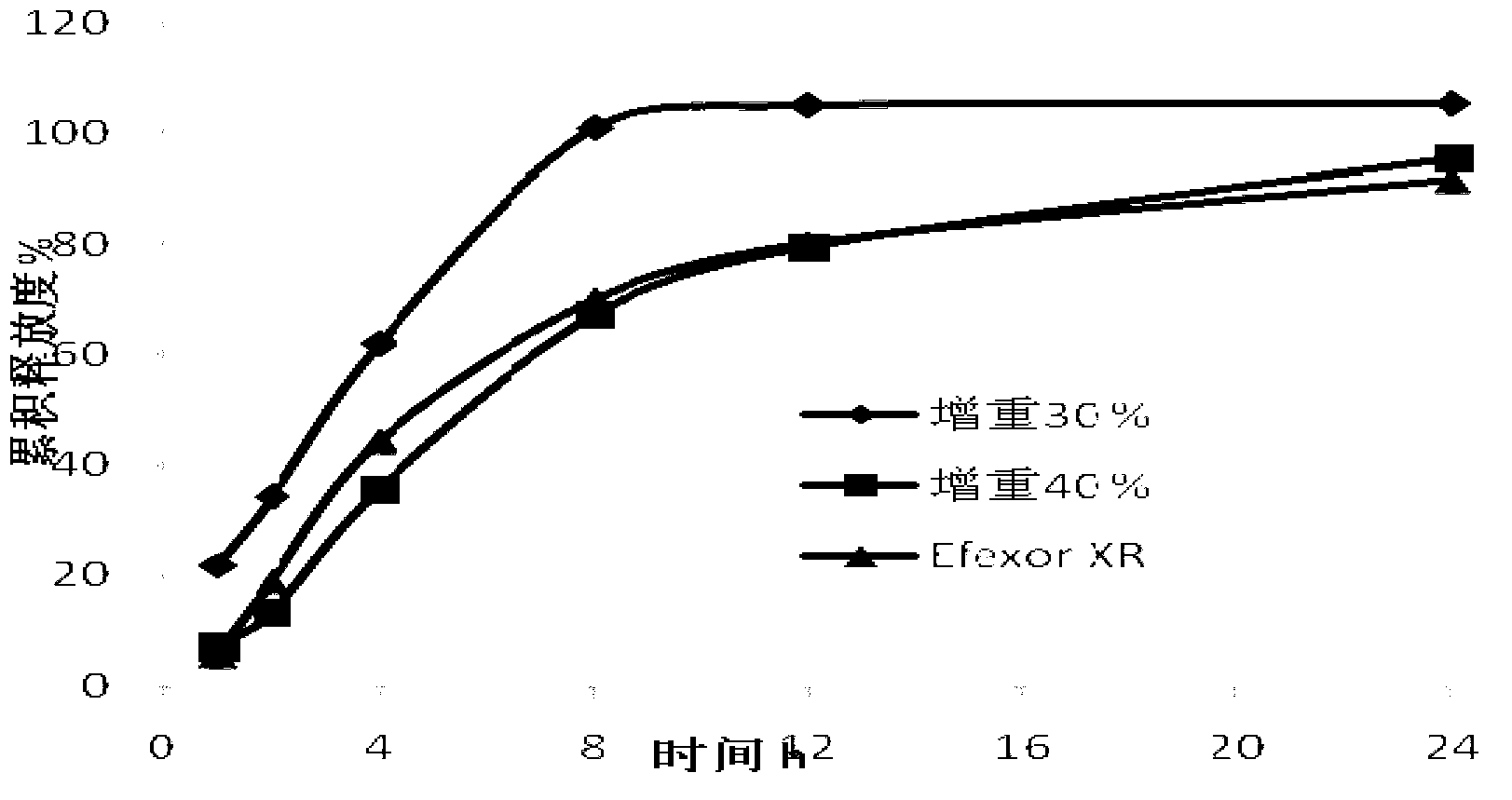
[0058] Coating operation: take 500 g of the 30-16 mesh pellet cores prepared in Example 3, and coat the isolation layer and the release-controlling layer respectively with the method of bottom spraying to prepare sustained-release pellets. Coating weight gain (calculated by polymer weight) 30%, 40%.
[0059] Release degree inspection: According to the Chinese drug import registration standard X20000237, the release degree of the product was inspected, and compared with Efexor XR, a product of Wyeth Pharmaceutical Co., Ltd.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com