Medicament for preventing and treating viral hepatitis of ducklings
A viral hepatitis and drug technology, applied in the field of medicine and medical engineering, can solve the problems of imprecise formula, poor efficacy, food safety threat, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Test time: December 2006.
[0015] The test location: Fanjingshan Duck Farm, Shahe City, Hebei Province.
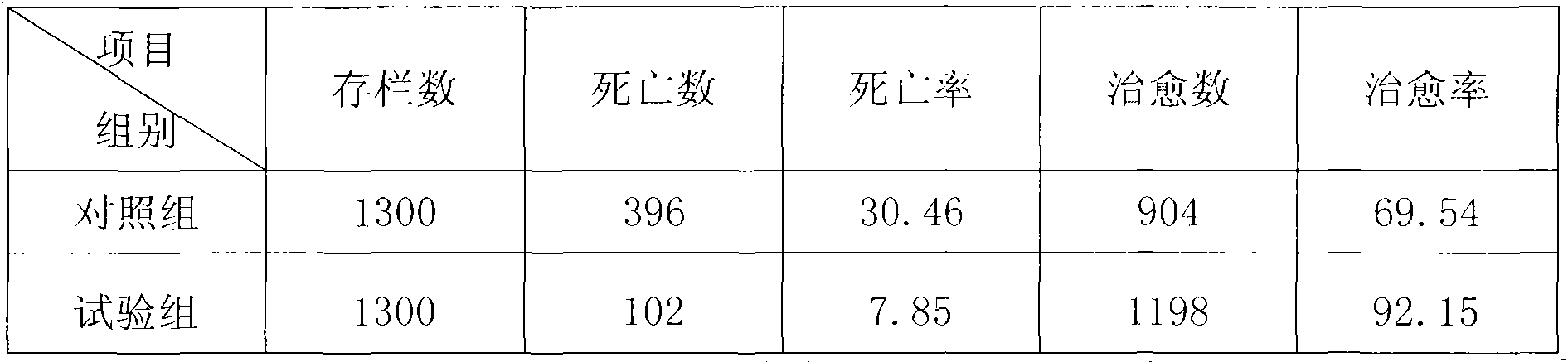
[0016] The tested duck group: 18 days old, 2600 Kangbei commercial laying ducks, due to the lack of vaccination, cold weather, poor ventilation and other factors, outbreaks of viral hepatitis in ducklings, all in the same group.
[0017] Drug for testing: the pure Chinese herbal medicine preparation of the present invention, each raw material drug is purchased from Dongfang Medicine City, Anguo City, Hebei Province, and its composition is expressed in parts by mass: 5 parts of Gentiana, 5 parts of Flos Lonicerae, 5 parts of Rhizome , 5 parts of Prunella vulgaris, 4 parts of Uncaria, 4 parts of Notopterygium, 4 parts of Duhuo, 4 parts of capillary, 3 parts of Paeoniae Alba, 3 parts of Turtle Shell, 3 parts of Heliconia, 3 parts of Qingpi, 2 parts of Rhubarb, 2 parts of Gardenia , 2 parts of jujube, 2 parts of licorice. The above-mentioned Chinese herbal medicine i...
Embodiment 2
[0031] Test time: March 2009.
[0032] Test site: Song Binbin Duck Farm, Linzhang County, Hebei Province.
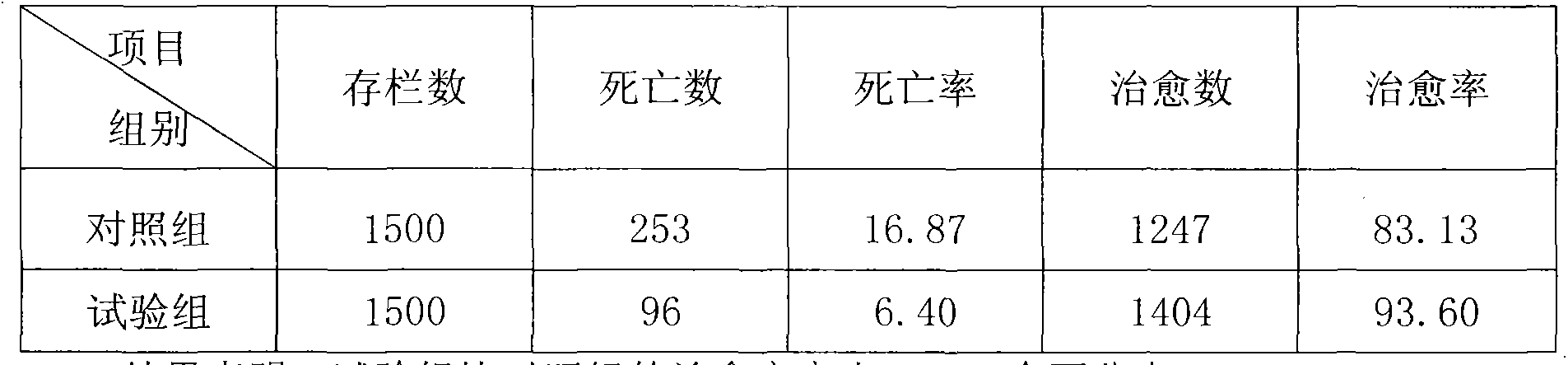
[0033] Tested duck group: 27 days old, 3,000 commercial cherry-grain egg ducks, due to factors such as lack of immunization, climate change, and improper management, duckling viral hepatitis disease broke out, and they were housed in the same group.
[0034] Drug for testing: namely the pure Chinese herbal medicine preparation of the present invention, the procurement of raw materials is the same as in Example 1, and its composition is expressed in parts by mass: 15 parts of Gentiana, 15 parts of Flos Lonicerae, 15 parts of Rhizome Rhizoma, 15 parts of Prunella vulgaris, 12 parts of rattan, 12 parts of Qianghuo, 12 parts of Duhuo, 12 parts of capillary, 10 parts of white peony root, 10 parts of turtle shell, 10 parts of scorpion, 10 parts of green bark, 6 parts of rhubarb, 6 parts of gardenia, 6 parts of jujube, licorice 6 servings. Processing method is with embodiment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com